Feline renal transplantation
Written by Lillian R. Aronson
Kidney transplantation has been pioneered in the USA as an option for treating feline renal disease and is still very much a specialist procedure, but Lillian Aronson reviews the technique, the ethics and the possible pitfalls in a paper that will be of benefit to all first-line small animal clinicians.

Key Points
La tosse nel cane anziano spesso correlata a condizioni come infiammazione delle vie aeree, collasso delle vie aeree, o bronchiectasia.
La diagnosi definitiva pu richiedere lesecuzione di test ematologici, radiografie, e un prelievo dalle vie aeree sotto anestesia.
Le malattie croniche richiedono solitamente una gestione a lungo termine e possono essere controllate con efficacia variabile, ma sono raramente curate.
In alcuni casi, sono necessari farmaci antitosse, ma questi possono talvolta intrappolare le secrezioni e peggiorare la malattia.
Introduction
Renal transplantation for cats continues to gain acceptance as a treatment option for patients presenting with early, decompensated chronic renal failure as well as those with acute irreversible renal failure. Since its introduction to veterinary medicine in 1987, it is estimated that between 600-700 cases of feline renal transplantation have been performed at various centers in the USA. The ability to successfully perform the technique in cats has been attributed to a number of factors, including the use of the drug cyclosporine for immunosuppressive therapy, the development and refinement of specific microsurgical techniques for the procedure, and the use of an allograft from an unrelated or related donor [1]. Successful transplantation can result in the disappearance of clinical signs previously associated with the patient’s kidney disease, weight gain, an overall improvement in the quality of life and prolonged survival time compared to medical management of the condition [2].
Client education
It is important for owners to understand that kidney transplantation in cats involves a long-term financial (and often emotional) commitment, as well as an obligation to ongoing postoperative management that should not be underestimated. The goal of the procedure is to improve the quality of life for the patient, but it is not a cure and complications can occur. Additionally, although medical treatments – including subcutaneous fluids, a renal diet, hormonal therapy to stimulate red blood cell production, phosphate binders, hypertension medication and gastrointestinal protectants – can often be discontinued following transplantation, lifelong immunosuppressive therapy is necessary to prevent rejection of the allograft. The owner needs to be informed about the risks of the procedure and should be alerted to the fact that their pet may be turned down as a potential candidate if the patient is fractious or if it fails any aspect of the medical screening process. Financial commitments include the costs incurred from both the recipient and donor during their initial hospitalization, as well as additional expenses once the patient leaves the transplant facility. These include repeated veterinary visits to perform routine blood work and wellness examinations, and also the cost of treating potential complications if they arise. Prior to the procedure, the owner should identify a veterinarian and facility committed to performing long-term follow-up and willing to provide 24-hour care should complications develop. Additionally, regardless of the outcome, the owner is responsible for adopting the donor cat and providing the animal with a lifelong home.
Recipient and donor screening
Recipient
Thorough screening of a potential recipient can prevent complications from occurring following the procedure, and is typically performed by the referring veterinarian in conjunction with the transplant team. Cats should be free of other disease conditions including recurrent urinary tract infections, significant heart disease, feline leukemia virus [FeLV] or feline immunodeficiency virus [FIV] positive status, and underlying neoplasia. Note that calcium oxalate urolithiasis, inflammatory bowel disease and/or a history of an upper respiratory infection are not contraindications to performing this procedure, as cats diagnosed with these conditions have been successfully transplanted at the author’s facility [3].
|
Table 1. Preoperative evaluation for a potential feline renal transplant recipient.
Although the exact time to intervene is still up for debate, the author recommends surgical intervention for patients with irreversible acute renal failure or those with chronic disease showing signs of decompensation, including continued weight loss and worsening of the azotemia and anemia in the face of medical management [4] [5]. It is important to note that clinically stable patients can rapidly deteriorate and die without prior evidence that decompensation was present. Although there is no set age limit for the procedure, recipient age has been associated with survival following discharge, with one study identifying cats older than 10 years of age having a higher-mortality rate during the first 6 months after surgery and another study identifying a decrease in median survival time with increasing age [2] [6].
Current evaluation of a potential recipient involves laboratory testing (blood type and cross-match, complete blood count [CBC] and biochemistry, and thyroid evaluation), urinary tract evaluation (urinalysis, urine culture, urine protein:creatinine ratio, abdominal radiography, and abdominal ultrasonography), cardiac evaluation (thoracic radiography, electrocardiography, echocardiography and blood pressure) and screening for infectious disease (FeLV, FIV], as well as serologic testing for toxoplasmosis [IgG and IgM]) (Table 1). If a patient is traveling from a significant distance, a blood sample can be sent for cross-matching to potential donors prior to travel to determine if a compatible donor is available.
Donor
The author’s facility maintains a donor colony that consists of healthy young (typically 1-3 years old) cats adopted from a regional shelter. Once a donor is identified for a specific recipient, the owner of the recipient is responsible for adopting the donor cat and giving him/her a lifelong home. The wellbeing of the donor cat is of the utmost importance to the transplant team. Those who work in the field of transplantation understand the ethical implications of the procedure, including concerns that have been raised by ethicists regarding possible harm and suffering that could be inflicted from the procedure, as well as any effect on long-term survival. Because of these concerns, a large retrospective study was performed at the author's facility in 2016 looking at perioperative morbidity and long-term outcome following unilateral nephrectomy in 141 kidney donors [7]. The study identified an acceptably low perioperative morbidity, and the median time from nephrectomy to hospital discharge was 3.6 days. Long-term follow-up was available for 99 cats; the median age at the time of follow-up was 12.2 years. Three cats had a history of stable chronic kidney disease (median 6.2 years post-operatively) and two cats were treated successfully for acute kidney injury, 4 and 6 years following surgery. Two cats died of chronic renal failure 12 and 13 years following surgery and four cats developed an acute ureteral obstruction from calcium oxalate urolithiasis a median of 7 years following surgery. Because of this latter finding, routine abdominal radiographs are performed during yearly wellness visits to identify any new stone formation so that it can be addressed accordingly before resulting in morbidity or mortality.
Standard donor evaluation includes CT angiography to assess the renal vasculature as well as to evaluate the renal parenchyma for any abnormalities (Figure 1a) (Figure 1b). Other mandatory tests include a complete blood count and blood type, serum biochemistry profile, urinalysis and culture, FeLV and FIV testing and serologic testing for toxoplasmosis (IgG and IgM). A suitable home is found for any potential donor that fails the screening process.
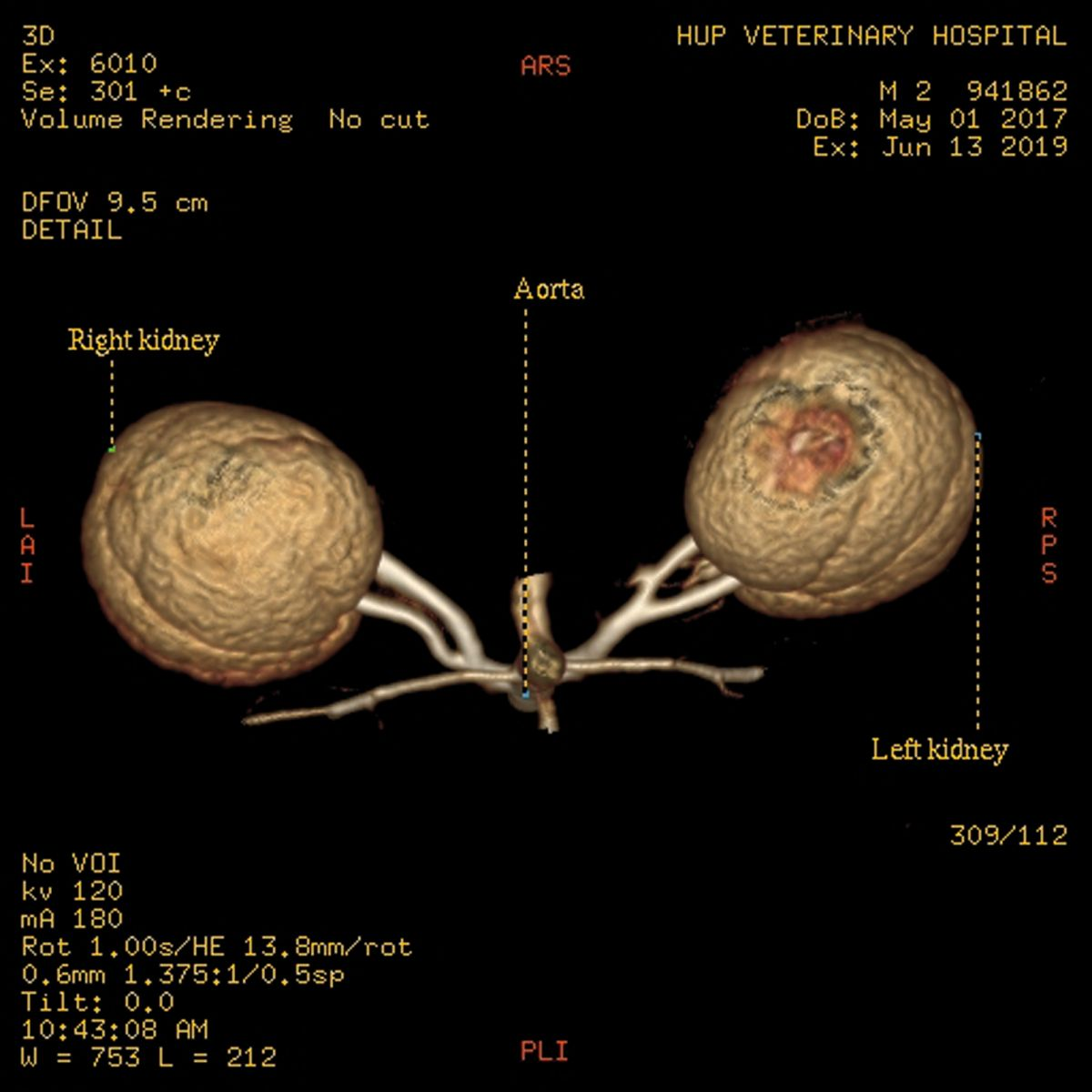
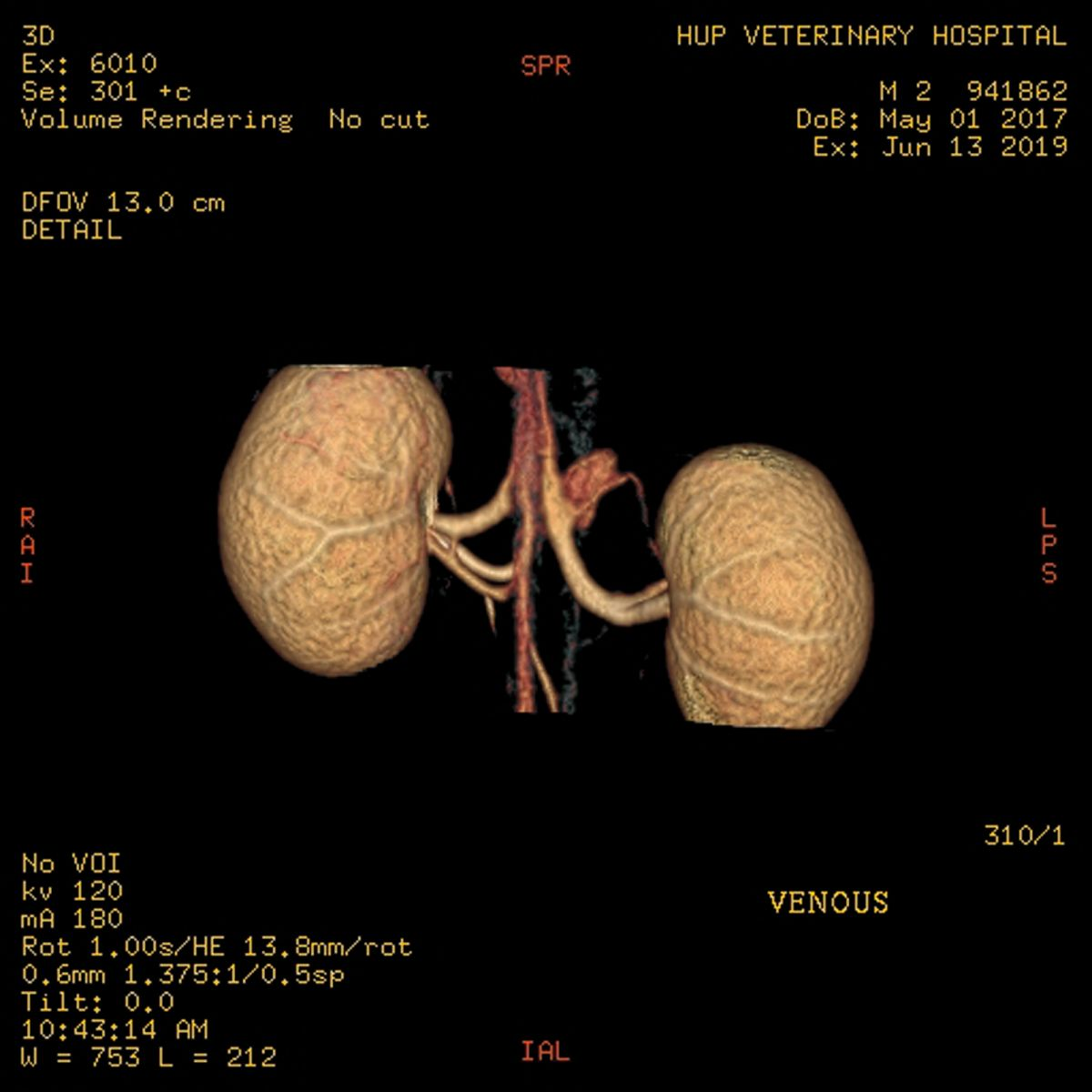
Preoperative treatment
Medical management – including an appropriate renal diet, fluid therapy, blood products, gastrointestinal protectants, phosphate binders and antihypertensive therapy – will vary depending on the stability of the recipient. If the patient is anorectic, a nasogastric tube is placed to administer nutritional support and prevent hepatic lipidosis. However, because of complications encountered with esophagostomy tubes in some recipients receiving chronic immunosuppressive therapy, such tubes are no longer recommended by the author in this population of patients unless absolutely necessary.
Successful renal transplantation can result in the disappearance of clinical signs previously associated with the patient’s kidney disease, weight gain, an overall improvement in the quality of life and prolonged survival time compared to medical management of the condition.
Two immunosuppressive protocols currently exist to prevent rejection of the allograft. The protocol currently used at the author’s facility includes a combination of the calcineurin inhibitor cyclosporine (CsA) and the corticosteroid, prednisolone. An oral liquid formulation of cyclosporine is used so that the dose can be titrated for each individual cat. Cyclosporine is typically begun 72-96h prior to transplantation whilst prednisolone administration is started on the morning of surgery. A 12-hour whole-blood cyclosporine trough concentration is obtained the day before surgery to adjust the oral dose for surgery. Vitamin B12 (given by intramuscular injection) has been used in some patients to aid cyclosporine absorption from the gastrointestinal tract. Another option for immunosuppression used by some transplant surgeons combines ketoconazole with cyclosporine and prednisolone, allowing for once daily administration of medication [8] [9]. With this protocol, cyclosporine trough concentration is measured every 24 hours. If signs of hepatotoxicity are identified, ketoconazole administration should be discontinued. If the cat is positive on IgM and IgG serology for Toxoplasma gondii, clindamycin is administered in conjunction with immunosuppressive therapy and continued for the lifetime of the cat.
Surgery
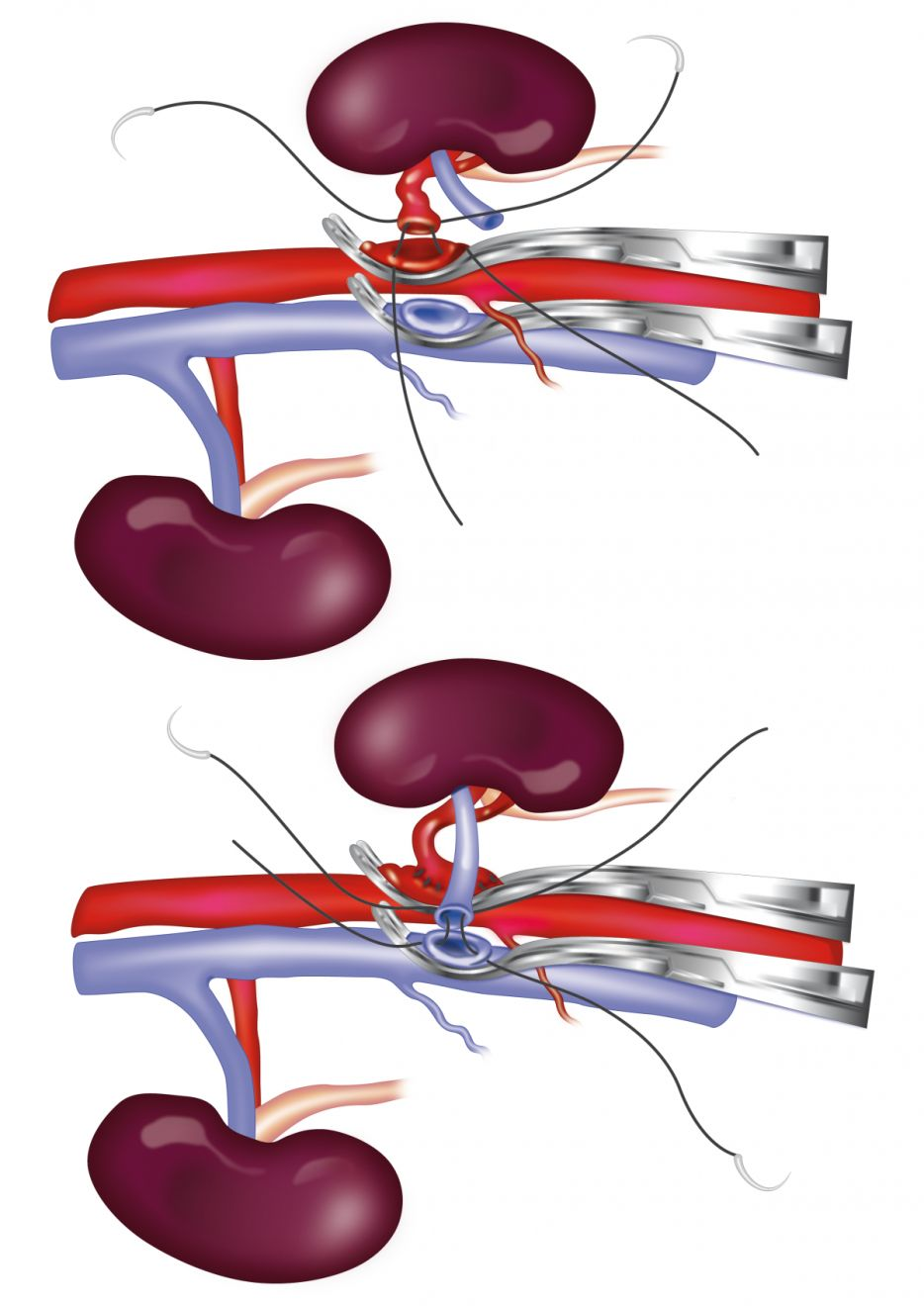
At the author’s facility, the transplant procedure takes approximately 6-7 hours and involves a team of 3 surgeons. The donor kidney is prepared for nephrectomy. The left kidney is preferred because it has a longer vein, however the right kidney can be used if necessary. The renal artery and vein are cleared of fat and adventitia and the ureter is dissected free to the point where it joins the bladder. It is critical, however, to harvest a donor kidney with a single renal artery, with a minimal length of 0.5 cm at the point where the artery joins the aorta [10]. The nephrectomy is performed when the recipient’s vasculature is prepared to receive the kidney.
An operating microscope is used to perform the majority of the recipient’s surgery. The allograft is flushed with a phosphate-buffered sucrose organ preservation solution or heparinized saline solution; the donor renal artery is anastomosed end-to-side to the abdominal aorta using 8-0 nylon in a simple continuous pattern, and the donor renal vein is anastomosed end-to-side to the caudal vena cava using 7-0 silk in a simple continuous pattern (Figure 2) [10]. Once complete, the hemostat clamps are removed; a small amount of hemorrhage usually occurs at this point and is controlled with pressure, but any significant leaks may need to be repaired with the placement of additional sutures.
An alternative surgical technique uses hypothermic storage to preserve the donor kidney until the recipient surgery is performed. This technique reduces personnel and resources needed for the transplantation procedure.
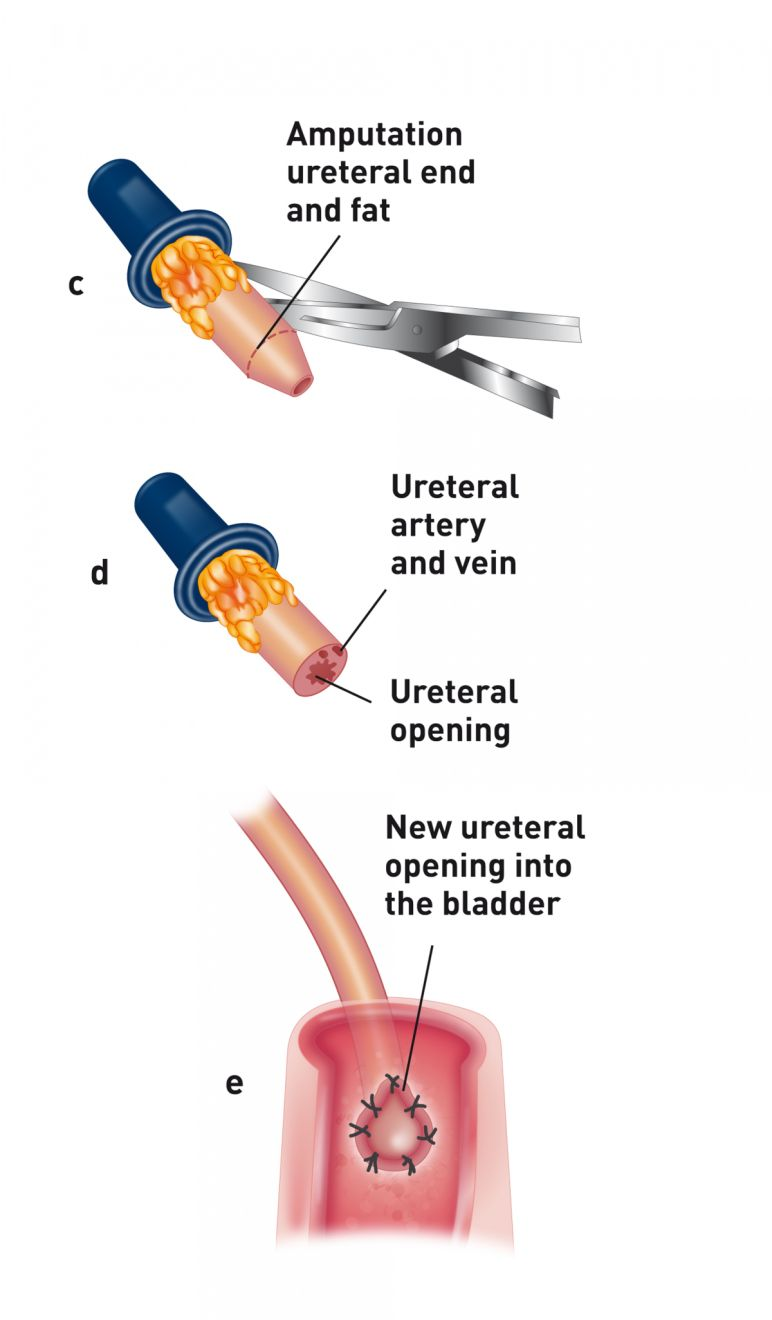
Following the vascular portion of the procedure, the donor ureter is attached to the urinary bladder using one of three techniques. At the author’s facility, a ureteroneocystostomy is performed using an intravesicular mucosal apposition technique. A ventral midline cystotomy is performed and then the end of the ureter is brought directly into the bladder at the apex. The end of the ureter is spatulated and the ureteral mucosa is sutured to the bladder mucosa using either 8-0 nylon or synthetic absorbable suture material in a simple interrupted pattern (Figure 3a) (Figure 3b). Prior to closure, the allograft is attached to the abdominal wall to prevent torsion. The recipient’s native kidneys are usually left in place to act as a reserve in case graft function is delayed (Figure 4). Because patients are on immunosuppressive therapy, non-absorbable suture (polypropylene) is used for abdominal wall closure to prevent incision dehiscence.

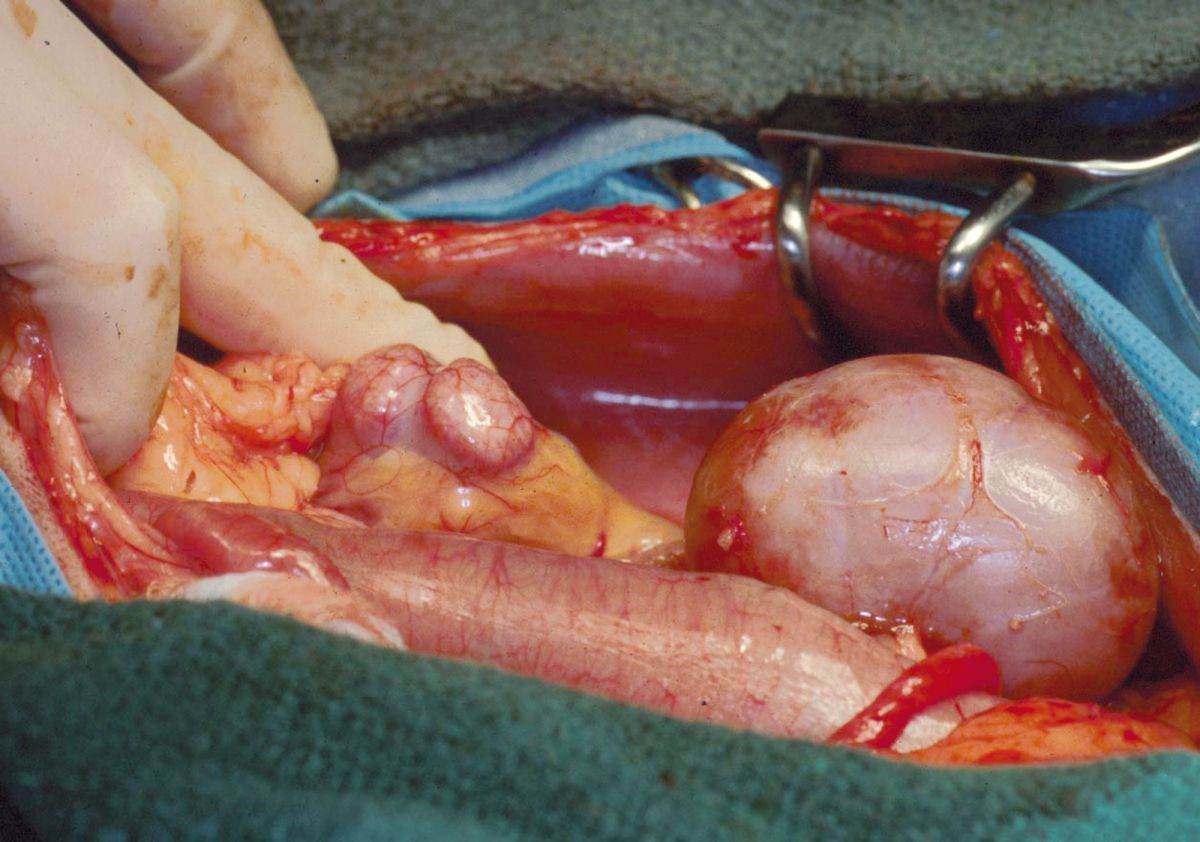
Postoperative care
Postoperative care is tailored for each individual patient, but typically includes intravenous fluid therapy until the patient is eating and drinking, antibiotic therapy, blood products as needed, and pain control. Minimal stress and handling and prevention of hypothermia are critical during the early postoperative period. The packed cell volume, total protein, electrolytes, blood glucose and acid base status are initially evaluated 2-3 times daily for the first few days and then daily until discharge. Indirect blood pressure is monitored every 2-4 hours for the first 48-72 hours to monitor for the development of hypertension. A renal panel is checked every 24-48 hours, and a cyclosporine level checked every 3-4 days and dosage adjusted accordingly. If needed, a full blood biochemistry and complete blood count is performed. Voided urine is collected daily to assess urine specific gravity. If the procedure is successful, azotemia typically resolves within the first 24-72 hours following surgery. If improvement is not identified, an ultrasonographic examination of the allograft is recommended to assess blood flow as well as for any signs of a ureteral obstruction. If graft perfusion is adequate and no signs of obstruction are present, then delayed graft function should be considered. In these cases, improvement in function often occurs within the first few weeks following the procedure. If the transplanted kidney fails to function, the kidney should be biopsied prior to re-transplantation.
Long-term management and complications
Patients are confined either to a room without furniture or in a larger dog crate during the early postoperative period to prevent any catastrophic injury to the allograft. Weekly evaluations for the first 6-8 weeks are recommended, and then visits are extended to longer intervals depending on the animal’s stability. Long-term patients are evaluated by their veterinarian 3-4 times a year. During each exam, body weight and blood pressure are recorded. Clinical pathological evaluation should include a renal panel, packed cell volume, total protein, cyclosporine level and urinalysis if a free-catch urine sample is available. If indicated, a complete blood count and serum biochemistry panel is performed. Evaluation by a cardiologist is recommended every 6-12 months if the patient had been diagnosed with underlying cardiac disease prior to transplantation.
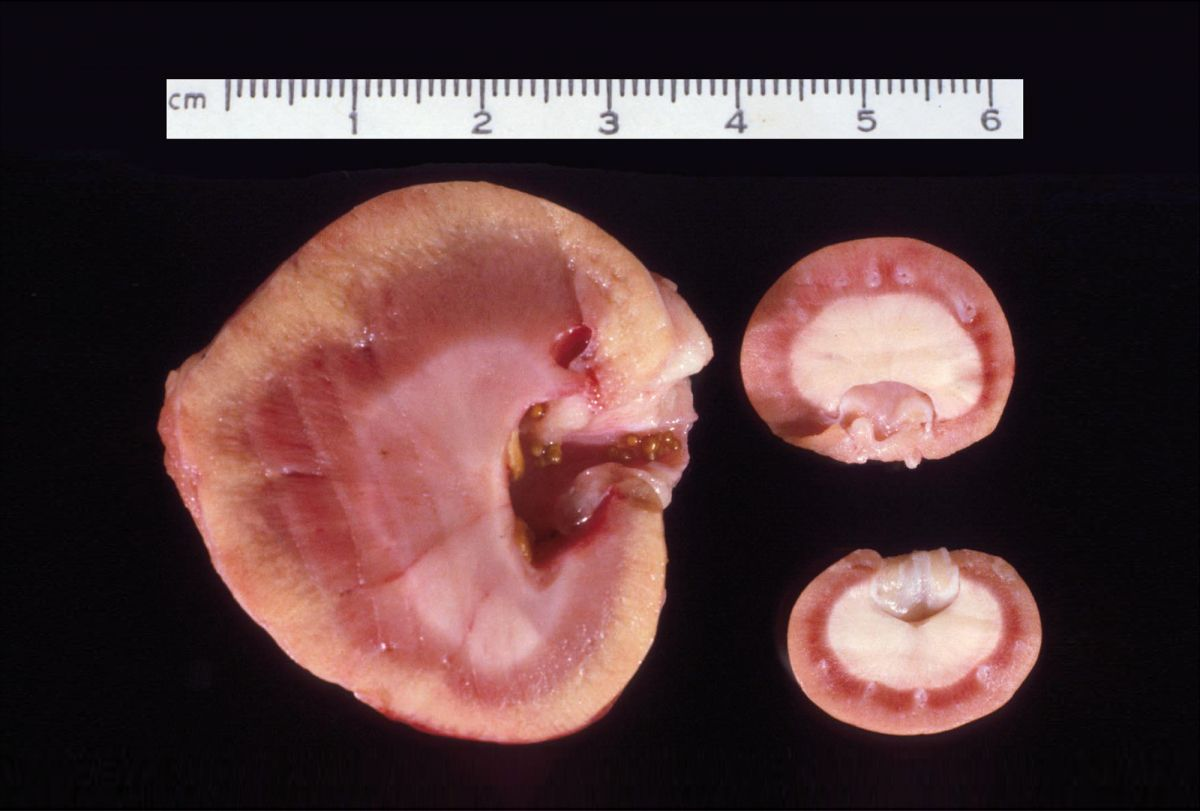
Complications from the procedure include those related to the allograft and those associated with chronic immunosuppressive therapy. Technical complications at surgery can occur with the vascular pedicle and ureteral reimplantation, resulting in the need for further procedures. Other complications directly related to the allograft include delayed graft function, acute rejection, calcium oxalate nephrosis (Figure 5), and retroperitoneal fibrosis (Figure 6a) (Figure 6b) [3] [11] [12]. Patients experiencing a rejection episode can be treated successfully with the administration of IV immunosuppressive therapy. Surgical intervention may be necessary for patients developing calcium oxalate urolithiasis of the allograft and is always indicated for cats developing retroperitoneal fibrosis in order to remove scar tissue that has resulted in a ureteral obstruction. Complications secondary to chronic immunosuppressive therapy include the development of infection (including opportunistic infections), diabetes mellitus (DM) and lymphoma (Figure 7) [13] [14] [15] [16] [17] [18] [19] [20]. Successful treatment of infectious complications is directed towards the specific infectious agent. For patients developing DM secondary to chronic immunosuppressive therapy, treatment involves an attempt to decrease the immunosuppressive drugs, dietary management and, in some cases, insulin administration. Unfortunately, treatment for patients who develop lymphoma following immunosuppressive therapy and transplantation has not been successful, and the prognosis is considered guarded.
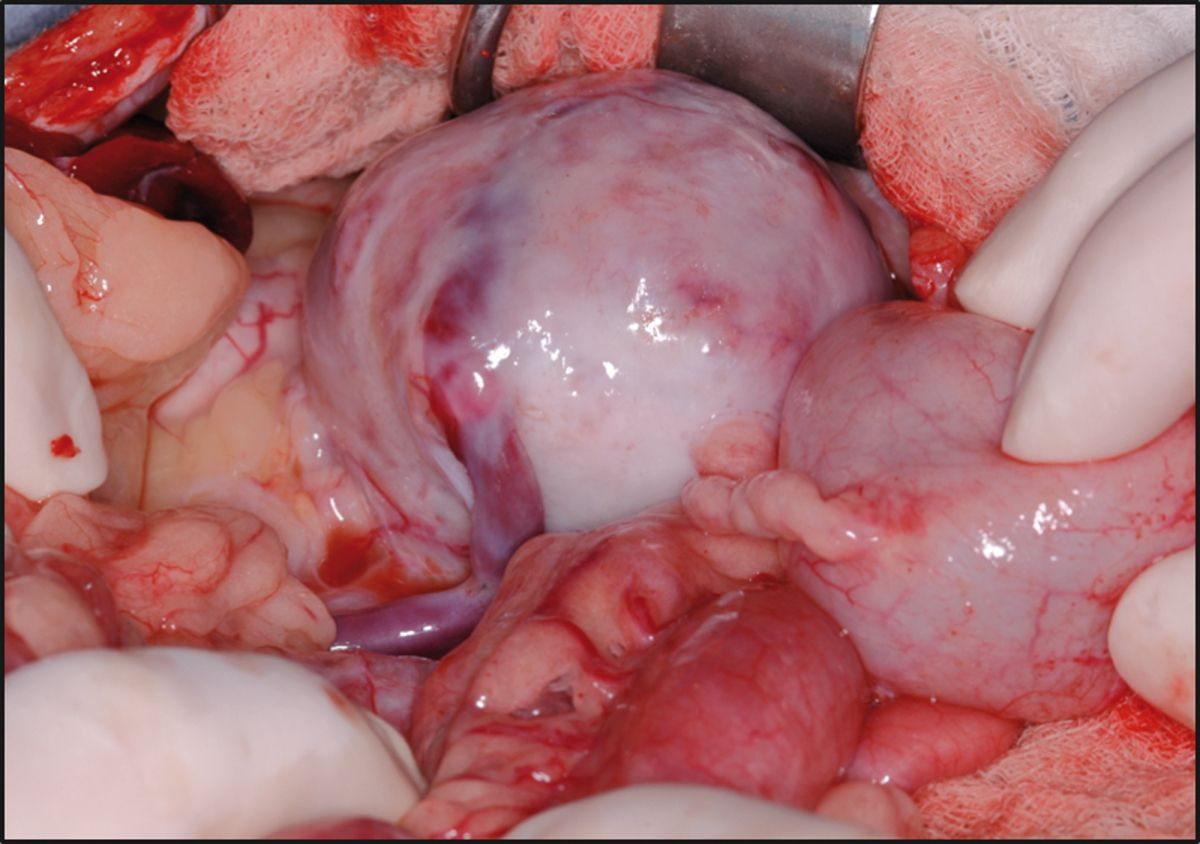
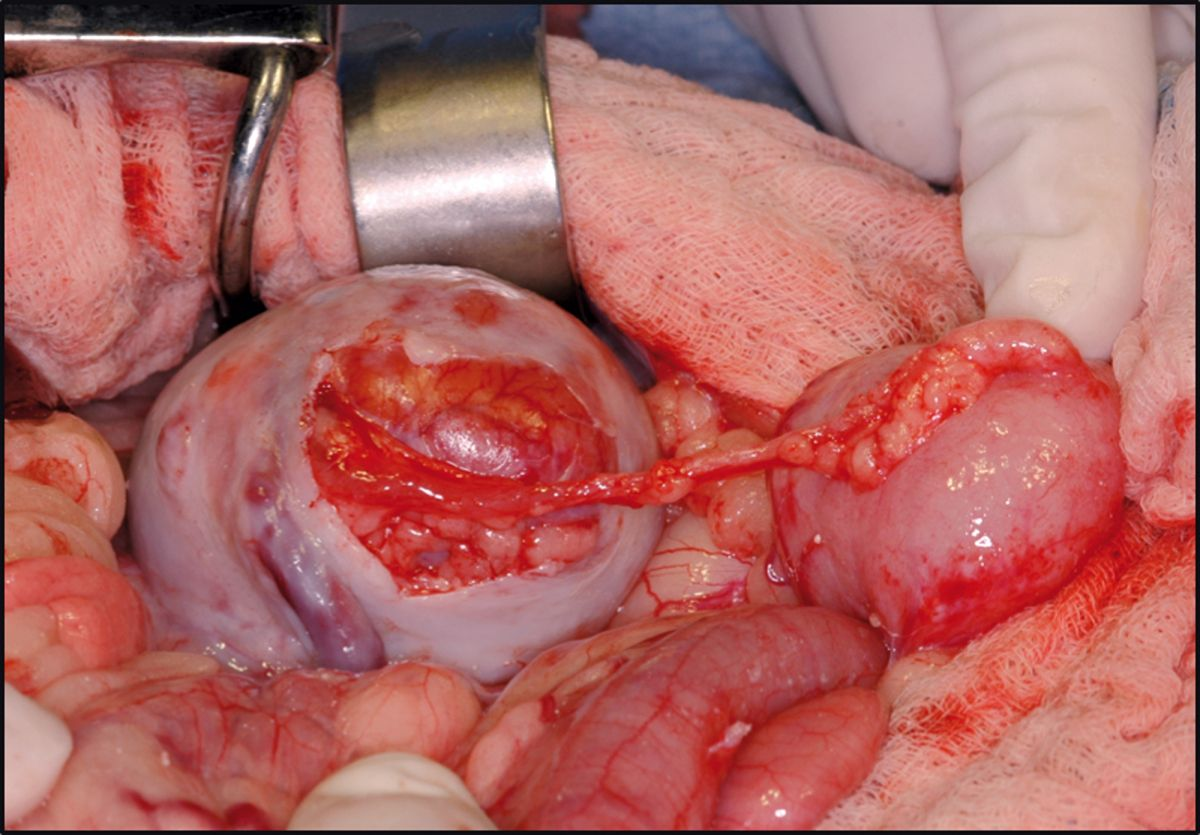

Currently at the author’s facility 92% of cats (154/168) have been discharged following surgery with a mean and median survival time of 994 and 595 days respectively. Ongoing clinical experience with short- and long-term management, as well as the ability to identify specific risk factors both pre- and post-operatively, will hopefully continue to improve long-term outcome in these patients. Whilst not an option for all cats with CKD, renal transplantation is becoming more widely available and the first-opinion practitioner should be aware of the pros and cons of the procedure, as well as the ethical and financial considerations that come with it.
Lillian R. Aronson
VMD, Dip. ACVS, USA
United States of America
After completing veterinary school and an internship at the University of Pennsylvania, Dr. Aronson undertook a small animal surgical residency at the University of California, Davis (UCD). From 1994-1996 she was the coordinator of the renal transplantation program for animals at UCD. Following her residency, she joined the faculty at the University of Pennsylvania – where she is currently Professor of Surgery – and started their renal transplantation program. Her clinical interests include all areas of soft tissue surgery, but in particular microvascular surgery and complex urinary tract surgery (including renal transplantation), and treatment of urolithiasis. As well as frequently lecturing in her specialist fields, she is the author of a textbook on small animal surgical emergencies.
References
- Gregory CR, Gourley IM. Organ transplantation in clinical veterinary practice. In: Slatter DH, ed. Textbook of Small Animal Surgery. Philadelphia: WB Saunders, 1993;95-100.
- Schmiedt CW, Holzman G, Schwarz T, et al. Survival, complications and analysis of risk factors after renal transplantation in cats. Vet Surg 2008;37:683-695.
- Aronson LR, Kyles AE, Preston A, et al. Renal transplantation in cats diagnosed with calcium oxalate urolithiasis: 19 cases (1997-2004). J Am Vet Med Assoc 2006;228:743-749.
- Gregory CR, Bernsteen L. Organ transplantation in clinical veterinary practice. In: Slatter DH, ed. Textbook of Small Animal Surgery. Philadelphia: WB Saunders, 2003;122-136.
- Mathews KG. Renal transplantation in the management of chronic renal failure. In: August J, ed. Consultation in Feline Internal Medicine 4. Philadelphia: WB Saunders, 2001;319.
- Adin CA, Gregory CR, Kyles AE, et al. Diagnostic predictors and survival after renal transplantation in cats. Vet Surg 2001;30:515-521.
- Wormser C, Aronson LR. Perioperative morbidity and long-term outcome of unilateral nephrectomy in feline kidney donors: 141 cases. J Am Vet Med Assoc 2016;248:275-281.
- Katayama M, McAnulty JF. Renal transplantation in cats: techniques, complications, and immunosuppression. Compend Contin Educ Pract Vet 2002;24:874-882.
- McAnulty JF, Lensmeyer GL. The effects of ketoconazole on the pharmacokinetics of cyclosporine A in cats. Vet Surg 1999;28:448-455.
- Aronson LR, Phillips H. Renal transplant. In; Johnston SA and Tobias KM, eds. Veterinary Surgery; Small Animal. St Louis: Elsevier, 2018;2263-2280.
- Aronson LR. Retroperitoneal fibrosis in four cats following renal transplantation. J Am Vet Med Assoc 2002;221:984-989.
- Wormser C, Phillips H, Aronson LR. Retroperitoneal fibrosis in feline renal transplant recipients: 29 cases (1998-2011). J Am Vet Med Assoc 2013;243:1580-1585.
- Kadar E, Sykes JE, Kass PH, et al. Evaluation of the prevalence of infections in cats after renal transplantation: 169 cases (1987-2003). J Am Vet Med Assoc 2005;227:948-953.
- Bernsteen L, Gregory CR, Aronson LR, et al. Acute toxoplasmosis following renal transplantation in three cats and a dog. J Am Vet Med Assoc 1999;215:1123-1126.
- Lo AJ, Goldschmidt MH, Aronson LR. Osteomyelitis of the coxofemoral joint due to Mycobacterium species in a feline transplant recipient. J Feline Med Surg 2012;14:919-923.
- Case JB, Kyles AE, Nelson RW, et al. Incidence of and risk factors for diabetes mellitus in cats that have undergone renal transplantation: 187 cases (1986-2005). J Am Vet Med Assoc 2007;230:880-884.
- Wooldridge J, Gregory CR, Mathews KG, et al. The prevalence of malignant neoplasia in feline renal transplant recipients. Vet Surg 2002;31:94-97.
- Durham AC, Mariano AD, Holmes ES, et al. Characterization of post transplantation lymphoma in feline renal transplant recipients. J Comp Pathol 2014;150:162-168.
- Wormser C, Mariano A, Holmes E, et al. Post-transplant malignant neoplasia associated with cyclosporine-based immunotherapy: prevalence, risk factors and survival in feline renal transplant recipients. Vet Compar Oncol 2016;14:e126-e134.
- Schmiedt CW, Grimes JA, Holzman G. Incidence and risk factors for development of malignant neoplasia after feline renal transplantation and cyclosporine-based immunosuppression. Vet Compar Oncol 2009;7:45-53.
Other articles on this issue
Share on social media