Small animal transfusion medicine
Written by João Araújo and Maria João Dourado
Confused about what blood product to use and when? This paper offers a review of the current options available for transfusion in small animal medicine.
Key points
The advent of commercial blood banks in many countries now means that transfusion of blood products can be done in most first opinion clinics.
Various blood products are obtained from whole blood, and the clinician should select the most appropriate product for each case.
Blood typing and cross matching are essential precursors to any blood product transfusion.
Transfusion reactions can occasionally occur, and patients must be carefully monitored before, during and after a transfusion.
Introduction
Transfusion medicine has a substantial place in small animal clinical practice, mainly in emergency and critical care situations, and the emergence of blood banks in many countries now permits easy access to, and rapid delivery of, various blood components to the clinic on demand. This, along with innovative and quick methods for blood typing and crossmatching, and recent advances in knowledge, means that transfusions can be done in most clinics. However, transfusion of blood products can be lifesaving, it can also potentially be life-threatening, and this article aims to review the current situation in order to provide useful information for the front-line practitioner.
Blood products
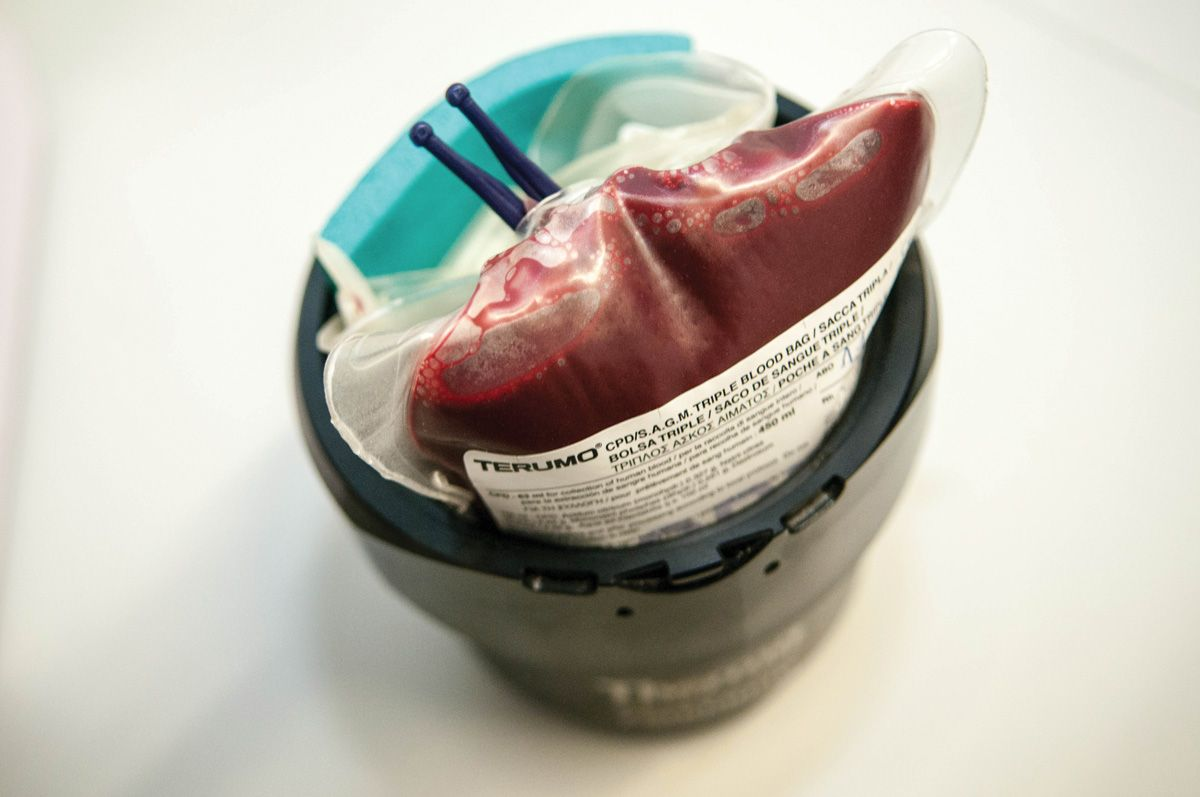
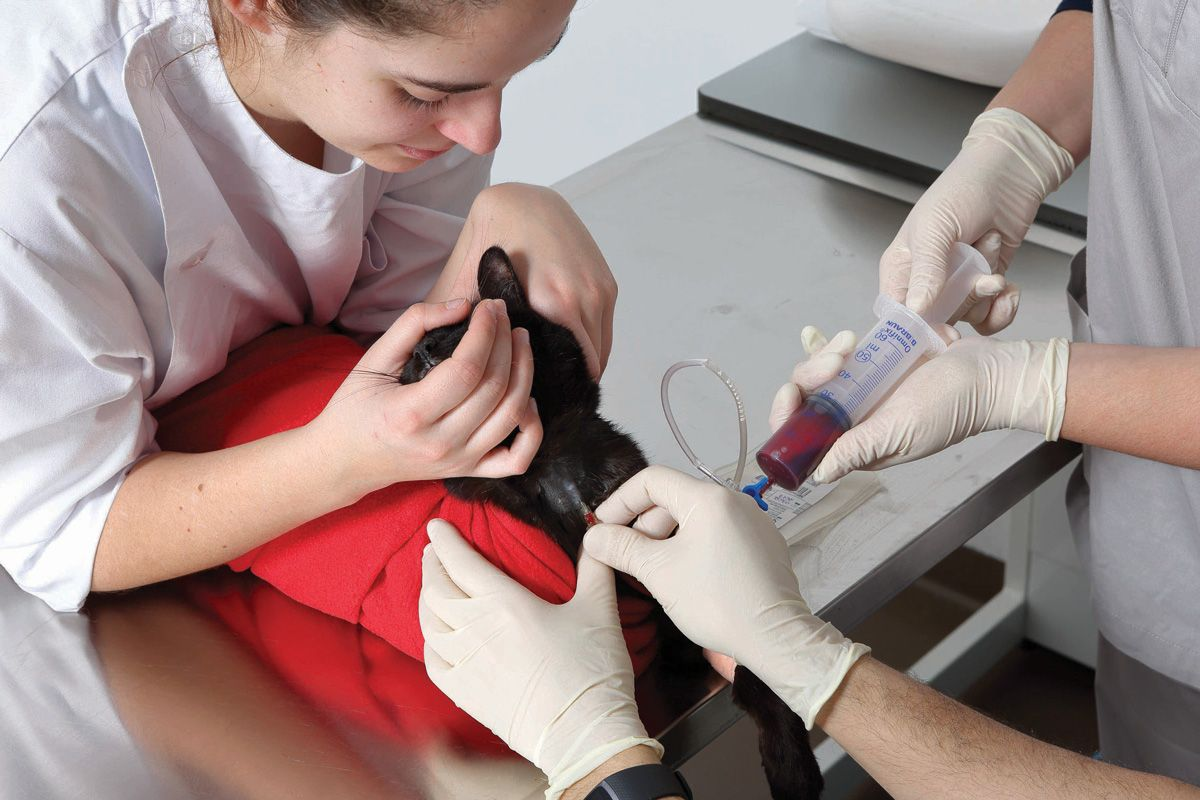
When blood is collected from a suitable donor, it is transferred to a bag or syringe containing anticoagulant and nutrients for the cells. Some veterinarians may choose to collect blood in their own clinic, using commercial collection bags; for dogs, these are typically supplied containing the correct amount of anticoagulant (usually CPD (Citrate-Phosphate-Dextrose)) for the volume of blood to be donated (Figure 1). When taking feline blood, a syringe should be pre-filled with 1 mL of anticoagulant for every 7 mL of blood to be collected (Figure 2). Alternatively, ready-to-use products can be purchased from dedicated blood banks (Figure 3), which offer several advantages; it allows the best product to be used for a specific condition; it reduces the risk of transfusion reactions; and it ensures efficient use of all blood resources [1], [2] . Several different products can be obtained from collected blood; these are broadly classified into red blood cell (RBC) products and plasma components, as shown in Box 1 [3].



Red blood cell products
- Fresh whole blood (FWB) – this contains red and white blood cells, platelets, coagulation factors, and plasma proteins such as albumin [4] . To be considered “fresh” it has to be transfused within 6-8 hours after collection [5] .
- Stored whole blood (SWB) – this is whole blood not transfused within the first 8 hours after collection, which means it has reduced levels of platelets and clotting factors. It should be stored in a refrigerator at 1-6ºC (33.8-42.8ºF), and can be used within 21-28 days of collection [3] ,[4] .
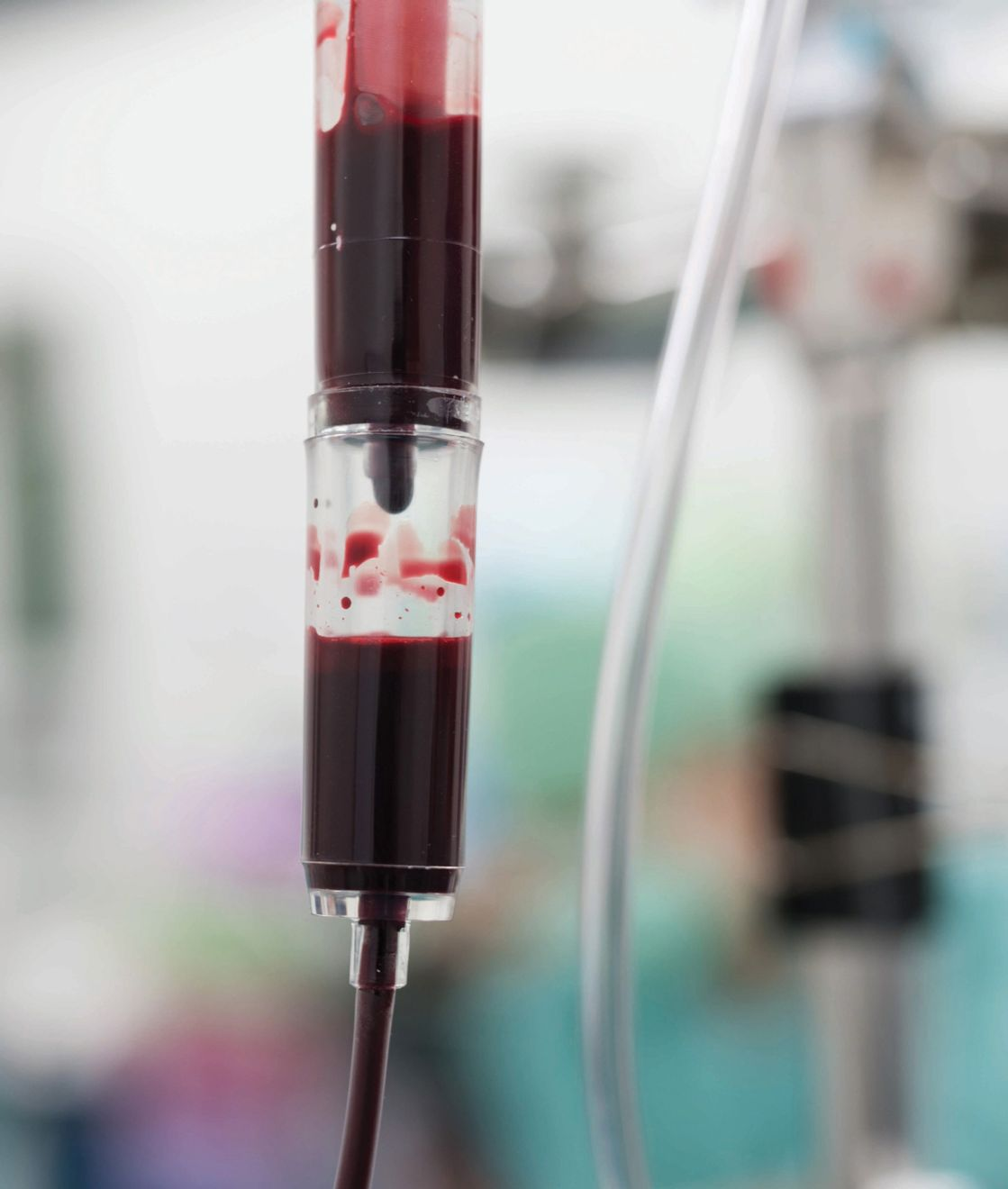
- Packed red blood cells (pRBC) – centrifugation of whole blood separates the erythrocytes from the plasma to produce a product with less oncotic pressure and no coagulation factors, and with a packed cell volume (PCV) of around 70-80% (Figure 4). If required, filtration will allow removal of the white blood cells to make the leukocyte-reduced pRBC [3] ,[4] , [5] .

Plasma products
These are obtained from whole blood centrifugation and contain all functional blood proteins at their original concentrations if performed within 8 hours of collection.
- Fresh frozen plasma (FFP) – Unless the fresh plasma is administered within 1 hour after processing [6], it should be frozen; to be classified as fresh frozen plasma, it must be frozen within 6-8 hours of collection, allowing the plasma to retain all the coagulation factors and proteins [3] (Figure 5).
- Stored frozen plasma (SFP) – Also simply designated as frozen plasma, this term is applied to fresh frozen plasma if it has gone past its expiry date, or where centrifugation or freezing is performed more than 8 hours after collection, or to FFP which has been thawed and then refrozen without being opened. SFP has fewer clotting factors and proteins than FFP [3] , [6].
- Cryoprecipitate and cryosupernatant – Cryoprecipitate is produced by controlled thawing of a FFP unit followed by centrifugation; this results in a concentrated product containing insoluble proteins, factor VIII, von Willebrand factor (vWF) and fibrinogen [1] . The supernatant removed from the preparation of cryoprecipitate is called cryosupernatant (or cryo-poor plasma); it lacks vWF, fibrinogen and insoluble proteins, but retains albumin, hemostatic proteins and immunoglobulins [6].
- Platelet-rich plasma (PRP) and platelet concentrate (PC) – Platelet products have a short life and limited demand, so blood banks only produce these to order. Platelet-rich plasma results from a gentle spin of fresh whole blood [1] (Figure 6), whilst platelet concentrate is obtained by centrifugation of PRP which “pellets” the platelets [7].


Indications
Anemia, coagulopathies, sepsis, disseminated intravascular coagulopathy (DIC) and specific factor deficiencies are some of the indications for transfusion with an appropriate blood product [3] , but the decision to proceed should be made after considering all potential risks and benefits. A careful evaluation should be performed to address the recipient’s transfusion needs and to choose the correct blood component if there is no indication for whole blood transfusion. This maximizes the use of a blood unit and reduces the risk of transfusion reactions [8],[9].
Red blood cell products
Correct tissue oxygenation depends on the hemoglobin levels in the circulation and the cardiac output [3]. Since almost all the oxygen in blood is carried by hemoglobin, the way to increase oxygen transport is through transfusion of a RBC product. Determining the packed cell volume (PCV)/hematocrit (HCT) and hemoglobin concentration (HGB) of the patient, along with a physical evaluation, will help the veterinarian to decide if an animal needs blood therapy [8]. Although there is no exact PCV value below which a patient is deemed to need a transfusion, anemia (from hemorrhage, hemolysis or ineffective erythropoiesis) is the main reason to perform RBC transfusion, with some studies suggesting that blood loss is the main indication for transfusion in dogs and cats [10]. The preferred options are FWB or pRBC. FWB has all the physiological blood components (functional platelets, plasma proteins, coagulation factors) and is an excellent choice for anemia cases where there is also a coagulopathy or thrombocytopenia [8]. In cases of massive hemorrhage (loss of > 50% of circulating volume), FWB is also the product of choice in order to restore the oxygen transport function and the oncotic pressure [11] .
The administration of pRBC is indicated when there is an anemia in a normovolemic patient (hemolytic or non-regenerative; either acute or chronic). Because pRBC has an HCT of 70-80% and a lower oncotic pressure then whole blood, it is less likely to cause fluid overload in normovolemic recipients [1] ,[11] . Packed red blood cells can also be used in hypovolemic cases, but since there is a need to increase oncotic pressure and replenish other blood elements, FFP should be transfused as well [8].
Plasma products
The use of plasma products has been reported in various situations, including treatment of hypotension and to give oncotic support, to restore clotting factors in coagulopathies, for internal bleeding and uncontrollable mucosal bleeding, and as supportive treatment in cases of sepsis, trauma and gastric-dilation-volvulus [12].
Fresh frozen plasma has been proven to retain the functionality of hemostatic proteins for a year [13] and is the main choice to treat coagulopathies [12]. Frozen/stored plasma can retain non-labile coagulation factors such as Vitamin K, and is indicated for the treatment of coagulopathies due to rodenticide (anticoagulant) toxicosis [1] .
Cryoprecipitate is the most indicated plasma product to provide vWF, for the treatment of hemophilia A and fibrinogen deficiency or dysfunction [6], whilst cryosupernatant is the most cost-effective option to treat vitamin K deficiency [6].
The use of plasma products has also been described in cases of hypoproteinemia, using FFP and cryosupernatant containing albumin. However, a dose of 20-25 mL/kg is required to raise albumin levels by 0.5 g/dL [6].
Platelet products are mainly recommended for hemorrhage situations secondary to severe thrombocytopenia or other thrombopathies. If the bleeding is caused by DIC or immune-mediated thrombocytopenia, platelet products can also be helpful, but are less beneficial, since transfused platelets can be quickly destroyed [14].
From the authors’ experience, with the increase in blood product distribution by blood banks, pRBC and FFP are the most readily available components and therefore the most used to fulfil all the needs listed above [6].
Ready-to-use blood products can be purchased from dedicated blood banks, which offer several advantages; it allows the best product to be used for a specific condition; it reduces the risk of transfusion reactions; and it ensures efficient use of all blood resources.
Blood types
Both dogs and cats have species-specific blood types, defined by antigenic proteins on the surface of the red blood cells, and which can induce adverse reactions when introduced in another patient’s circulation. For this reason, it is not enough to emphasize that only compatible blood should be administered to the patient, and all animals should be blood typed and/or cross-matched.
Dogs
The canine blood types are grouped using the DEA (Dog Erythrocyte Antigen) system, which originally included DEA 1.1, 1.2, 1.3, 3, 4, 5, 6, 7 and 8. Antigens 6 and 8 are no longer routinely identified due to the absence of typing sera, but a new antigen, Dal, was reported in 2007 [15] and more recently another two antigens have been identified – Kai 1 and Kai 2 [16]. DEA 1.1 has been shown to have the highest antigenicity, and is known to have autosomal dominance inheritance, so dogs are now classified as being DEA 1.1 positive or negative [17]. This antigen can precipitate a severe hemolytic reaction in DEA 1.1 negative dogs that receive a second transfusion due to previous sensitization (since there are no natural alloantibodies) [18], so all blood donors and recipients should be tested for DEA 1.1.
One study has reported acute hemolytic transfusion reactions (AHTR) after sensitization to the DEA 4 antigen, but since 98% of dogs carry this antigen, only the 2% of dogs that are negative for this antigen are at risk of AHTR, and only after a previous transfusion. The other DEA antigens (3, 5 and 7) appear to have low importance in clinical practice, with no documented transfusion reactions [19].
Cats
Feline blood is categorized using the AB system, with 3 main types (A, B and AB) described, type A being the most frequent one. Unlike DEA 1.1 in dogs, cats have naturally occurring alloantibodies that can be responsible for hemolytic reactions [20]. Recently, a new blood-group antigen was identified, known as Mik [21]. This has clinical relevance, since there is a report of a hemolytic transfusion reaction in a type A cat which had never previously received a transfusion [21]. With this in mind, a crossmatch should be performed in all cats before a transfusion [22].
Blood typing
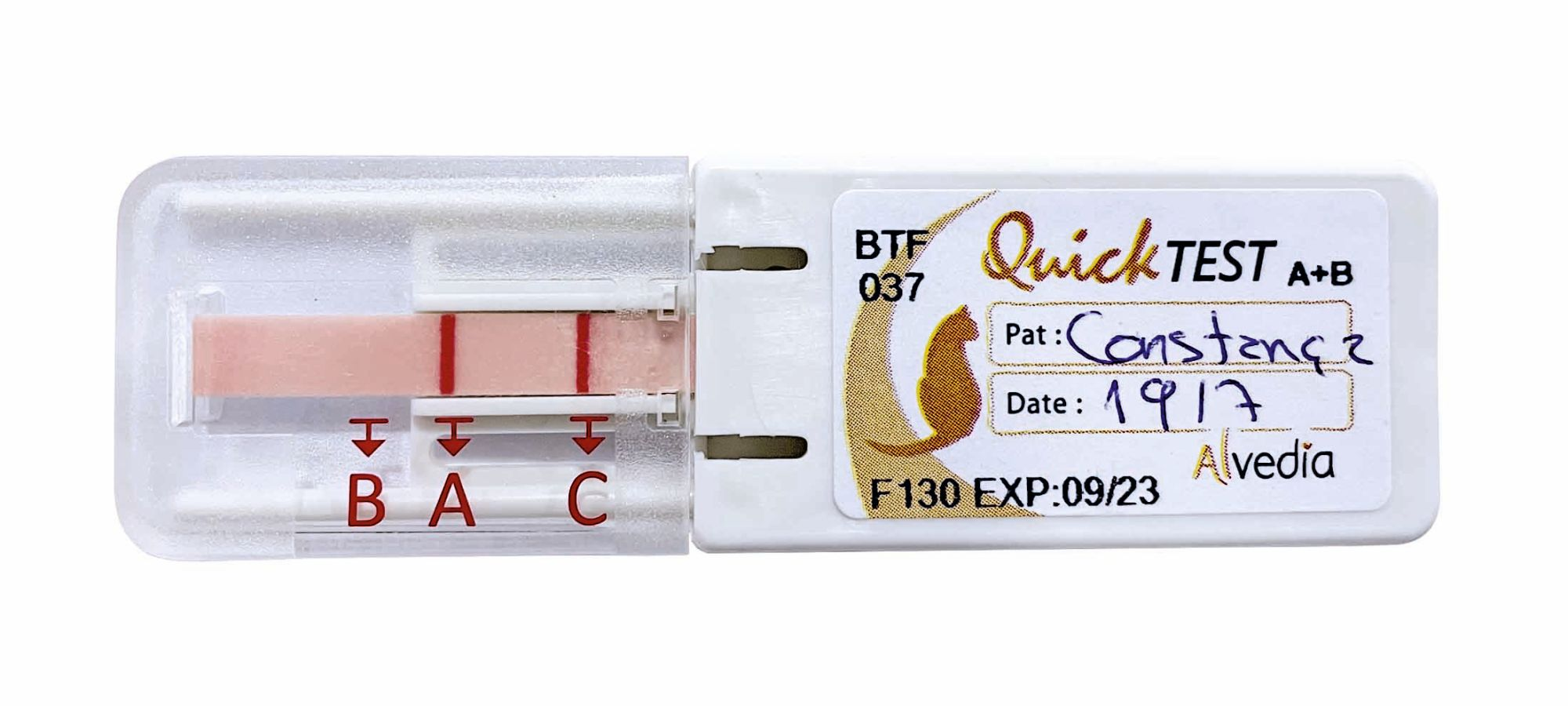
Blood typing can be done either at a commercial laboratory or in-house. There are currently three sorts of blood typing kits available: card agglutination, immune-chromatographic strip (ICS), and gel tube, but they all function in a similar way, with a sample of the patient’s blood being added to a mono- or polyclonal antiserum; a positive result (hemagglutination reaction) is identified by a color change (Figure 7). One study has reported that the card and ICS methods are a reasonable option for emergency situations, but the gel-based method appears to be the gold standard for typing DEA 1.1 donors and recipients [23].
Crossmatching
Whilst blood typing relates to antigens on the RBC, crossmatching – which can be done in-house – focuses on antibodies in the plasma, and can indicate if there may be a reaction between the blood of the donor and the recipient. It is a two-stage process, with a major and minor crossmatch [19]. The major crossmatch tests for compatibility between the donor erythrocytes and the recipient’s plasma; the minor one tests for compatibility between the donor plasma and the recipient’s erythrocytes. Any agglutination reaction implies incompatibility between donor and recipient.
The main indications for performing a crossmatching test in dogs are for where the patient has an unknown transfusion history, where it has had previous transfusion reactions, or if it has received a red blood cell transfusion more than 4 days previously [9]. As noted above, crossmatching should always be performed in cats.
At present, there are three types of tests available: the standard tube agglutination assay, the gel-tube assay and the immunochromatographic strip, with the first one currently being the gold standard [19].
How much to transfuse?
The volume of a blood product to be administered depends on various factors, including which product is being administered, what the desired effect is, and the patient’s response to the transfusion.
For pRBC’s, the following formula can be used:
|
Volume (mL)= 85 (dog) or 60 (cat) x bodyweight (kg) x [(Desired PCV - Actual PCV)/Donor PCV] |
When using a plasma product to treat hypotension, the recommended rate is 10 mL/kg over a period of 2-4 hours; the transfusion can be repeated every 6-24h as necessary. In severe cases, (e.g., refractory hypotension) the rate can be increased to 20-60 mL/kg. For severe hypoalbuminemia, a single transfusion at 10-20 mL/kg over 2-4 hours will increase albumin levels by 0.2 g/dL.
Plasma can also be administered in hypoalbuminemia patients using a constant rate infusion (CRI) of 1.5-3 mL/kg/hr over 12-24 hours to achieve an increment of 0.3-0.5 g/dL.
If platelet concentrate is being administered, 40-70 mL/10 kg SID-TID should be used until effect. Each transfusion should increase platelet number by 10-40 x 103/µL.
Administration of blood products can usually be done using only gravity flow, and infusion pumps should be generally avoided, especially with RBC products, since they may cause hemolysis.
Preparing and administering the blood product
When performing a transfusion, certain aspects may affect the quality of the blood product and the success of the transfusion. Firstly, before starting the procedure, it is important to evaluate the integrity of the storage bag, the color of the component and its consistency. Any unit with clots, an abnormal color or unusual appearance should not be used. A double check should be made that the correct unit will be given to the right animal.
Warming refrigerated RBCs is regarded as unnecessary in normovolemic and normothermic patients, since this may cause quicker deterioration of the cells and encourage microorganism growth. It should only be considered for hypothermic recipients, neonates or animals receiving a large amount of blood. Where warming is needed, the unit can be kept at room temperature for 30 minutes or placed inside a sealed plastic bag and put in a water bath (< 37ºC/98.6ºF) for 15 minutes [3],[8].
Frozen plasma products need to undergo a slow thaw before being administered to the patient; again placing the product inside a sealed bag before warming in a circulating water bath (< 37ºC/98.6ºF) is appropriate, with careful monitoring of the temperature [9].
The preferred route for blood product administration is the intravenous route, using a 20-22G catheter (which should be placed no earlier than 24h prior to the transfusion). The catheter should only be used for the transfusion; concomitant administration of medications, non-isotonic fluids or lactated Ringer’s should be avoided. If it is necessary to flush the catheter during the transfusion, this should be done using 0.9% saline. When the transfusion is finished, saline can then be attached to the perfusion set in order to avoid wasting the blood product that remains in the IV line. It is recommended to avoid giving antihistamines and antipyretics during the transfusion [22]. Where intravenous infusion is not possible (e.g., neonates), an intraosseous catheter can be used; cells will reach the blood circulation within minutes [4].
Infusions should be performed with a blood administration set that contains a filter (170-260 µm) which will retain clots and micro-aggregate particles [9], especially if whole blood is being transfused (Figure 8). If administering the blood product via a syringe pump, a pediatric filter with reduced dead space or a microaggregate filter of 18-40 µm should be used. Administration can usually be done simply using gravity flow, and infusion pumps should be generally avoided, especially with RBC products, since they may cause hemolysis [24]. If an infusion pump is necessary, the manufacturer’s instructions must be consulted to verify if the unit is safe for transfusion of blood products [8].
The rate of the transfusion depends on the clinical status of the patient. In a normovolemic animal the initial rate should be slow (0.25-0.5 mL/kg/hr) for the first 15-30 minutes to allow for any potential adverse reactions, before increasing to 2-10 mL/Kg/h in dogs and 3-5 mL/Kg/h in cats. An hourly rate of 10-20 mL/Kg is the maximum recommended in order to avoid fluid overload. Where this is a possibility (e.g., in cardiac or renal cases), the rate should be between 1-3 mL/Kg/h, starting at the lower end and increasing it to the maximum if there are no adverse reactions [1]. In cases that have severe blood loss and hypovolemia, a rapid volume correction may be tolerated using pRBC at 20-60 mL/Kg/h [8]. If higher rates are necessary, manual compression of the blood bag or a syringe bolus may be used.
Infusion times should not exceed four hours, as this increases the risk of bacterial contamination. In patients at risk of fluid overload, the recommended lower infusion rate may be incompatible with a four-hour window, and here the transfusion should be split, with refrigeration of the unused product as necessary [4].
Recipient monitoring
Monitoring the patient should begin before administration of any blood product, with evaluation of the PCV, total solids, heart rate, respiratory rate, mucous membrane color, body temperature and arterial blood pressure. The color of the urine can also be useful [4]. A physical exam should be performed every 15-30 minutes during the procedure and at 1, 12 and 24 hours after the end of the transfusion. The PCV, total solids and urine color should also be assessed immediately post-transfusion and then at 12 and 24 hours [3],[22]. In patients receiving a plasma product, coagulation status, hematocrit and/or total solids should be measured before and after transfusion, to verify the effectiveness of the therapy [6].
Adverse reactions
Various possible adverse reactions to transfusion of blood products are recognized. These include:
Febrile non-hemolytic transfusion reactions (FNHTR)
This is an acute reaction which can be either immunologic or non-immunologic in nature. Patients develop a temperature above 39ºC (102.2ºF) or have an increase in temperature more than 1ºC (1.8ºF) from that registered at the physical exam pre-transfusion. To diagnose a FNHTR, underlying infection, acute hemolytic transfusion reaction, transfusion-related acute lung injury and transfusion transmitted infection have to be excluded [25].
Respiratory reactions
These include Transfusion Associated Dyspnea (TAD), Transfusion-Associated Circulatory Overload (TACO), and Transfusion Related Acute Lung Injury (TRALI). TAD is again an acute transfusion reaction, with the patient developing severe respiratory distress within 24 hours following transfusion. For the correct diagnosis of TAD, transfusion circulatory overload and transfusion related acute lung injury must be ruled out [25]. TACO is an acute, non-immunologic reaction secondary to increased blood volume, with the patient showing signs of respiratory stress and pulmonary edema within 6 hours of transfusion [25]. TRALI is secondary to antigen-antibody interactions and is distinguished by acute hypoxemia and non-cardiogenic pulmonary edema, again within 6 hours of transfusion [25].
Allergic transfusions reactions
Acute and immunologic, these are secondary to a type I sensitivity response to an antigen. They are distinguished by an anaphylactic response (moderate to life-threatening) within 4 hours after transfusion. In the dog, clinical signs can include erythema, urticaria, pruritus, angioedema, gastrointestinal alterations and hemoabdomen with progression to collapse. In cats the signs are primarily respiratory, but pruritus and gastrointestinal signs are also reported [25].
Hemolytic reactions
These can be acute or delayed in nature. An AHTR is characterized by an acute hemolysis which can be immunologic or non-immunologic in nature; it is a non-infectious reaction occurring during or within 24 hours of the transfusion procedure [25]. Delayed reactions (24 hours to 28 days afterwards) are also non-infectious, and again can be immunologic or non-immunologic; they are secondary to lysis or accelerated clearance of transfused RBCs [25].
Conclusion
Blood transfusions were originally a treatment option that only referral centers could offer, but with the ready availability of blood products and the ability to perform in-house blood typing and crossmatching, most clinics can now offer transfusions with good quality blood products. Since blood products are a valuable and finite resource, it is important to use them rationally and minimize wastage; a transfusion is often not the only and definitive treatment. Optimizing use of the different blood products is essential, meaning that whole blood is often not the “go-to” product, so a correct diagnosis is necessary to ensure a good outcome. Finally, it is fundamental that the blood donor’s welfare is protected during the entire collection process.
References
- Davidow B. Transfusion medicine in small animals. Vet. Clin. North Am. Small Anim. Pract. 2013;43(4):735-756. https://doi.org/10.1016/j.cvsm.2013.03.007
- Logan JC, Callan MB, Drew K, et al. Clinical indications for use of fresh frozen plasma in dogs: 74 dogs (October through December 1999). J. Am. Vet. Med. Assoc. 2001;218(9):1449-1455. https://doi.org/10.2460/javma.2001.218.1449
- Gibson G, Callan MB. Transfusion medicine. In; BSAVA Manual of Canine and Feline Emergency and Critical Care. Gloucester, BSAVA; 2018;236-248. https://doi.org/10.22233/9781910443262.14
- Chiaramonte D. Blood-component therapy: selection, administration and monitoring. Clin. Tech. Small Anim. Pract. 2004;19(2):63-67. https://doi.org/10.1053/j.ctsap.2004.01.003
- Prittie JE. Triggers for use, optimal dosing, and problems associated with red cell transfusions. Vet. Clin. North Am. Small Anim. Pract. 2003;33(6);1261-1275. https://doi.org/10.1016/s0195-5616(03)00093-7
- Brooks MB. Transfusion of Plasma Products. In; Schalms Veterinary Hematology Brooks MB, Harr KE, Seelig DM, et al (eds). Hodboken, NJ; John Wiley Sons 2022;914-920. https://doi.org/10.1002/9781119500537.ch100
- Abrams-Ogg, ACG, Blois SL. Principles of Canine and Feline Blood Collection, Processing, and Storage. In; Schalms Veterinary Hematology. Brooks MB, Harr KE, Seelig DM, et al (eds). Hodboken, NJ; John Wiley Sons. 2022;898-907.
- Callan MB. Red Blood Cell Transfusion in the Dog and Cat. In; Schalms Veterinary Hematology. Brooks MB, Harr KE, Seelig DM, et al (eds). Hodboken, NJ; John Wiley Sons 2022;908-913. https://doi.org/10.1002/9781119500537.ch99
- Sink CA. Clinical Considerations in Transfusion Practice. In; Practical Transfusion Medicine for the Small Animal Practitioner. Hoboken, NJ: John Wiley Sons 2017;32-41 https://doi.org/10.1002/9781119187691.ch4
- Klaser DA, Reine NJ, Hohenhaus AE. Red blood cell transfusions in cats: 126 cases (1999). J. Am. Vet. Med. Assoc. 2005;226(6):920-923. https://doi.org/10.2460/javma.2005.226.920
- Lanevschi A, Wardrop KJ. Principles of transfusion medicine in small animals. Can. Vet. J. 2001;42(6):447-454.
- Elias Santo‐Domingo N, Lewis DH. Indications for use and complications associated with canine plasma products in 170 patients. J. Vet. Emerg. Crit. Care 2021;31(2):263-268. https://doi.org/10.1111/vec.13047
- Wardrop, KJ. Clinical Blood Typing and Crossmatching. In; Schalms Veterinary Hematology. Brooks MB, Harr KE, Seelig DM, et al (eds). Hodboken, NJ; John Wiley Sons. 2022;964-968.
- Abrams-Ogg ACG, Blois SL. Platelet and Granulocyte Transfusion. In; Schalms Veterinary Hematology. Brooks MB, Harr KE, Seelig DM, et al (eds). Hodboken, NJ; John Wiley Sons. 2022;921-926. https://doi.org/10.1002/9781119500537.ch101
- Blais MC, Berman L, Oakley DA, et al. Canine Dal blood type: a red cell antigen lacking in some Dalmatians. J. Vet. Int. Med. 2007;21(2);281-286.
- Lee JH, Giger U, Kim HY. Kai 1 and Kai 2: Characterization of these dog erythrocyte antigens by monoclonal antibodies. PLOS ONE, 2017;12(6), e0179932. https://doi.org/10.1371/journal.pone.0179932
- Polak, K, Acierno MM, Raj K, et al. Dog erythrocyte antigen 1: Mode of inheritance and initial characterization. Vet. Clin. Pathol. 2015;44(3);369-379. https://doi.org/10.1111/vcp.12284
- Giger U, Gelens CJ, Callan MB, et al. An acute hemolytic transfusion reaction caused by dog erythrocyte antigen 1.1 incompatibility in a previously sensitized dog. J. Am. Vet. Med. Assoc. 1995;206(9);1358-1362.
- Zaremba R, Brooks A, Thomovsky E. Transfusion medicine: an update on antigens, antibodies and serologic testing in dogs and cats. Topics Comp. Anim. Med. 2019;34:36-46. https://doi.org/10.1053/j.tcam.2018.12.005
- Hohenhaus AE. Importance of blood groups and blood group antibodies in companion animals. Transfusion Med. Rev. 2004;18(2):117-126.
- Weinstein, NM, Blais MC, Harris K, et al. A newly recognized blood group in domestic shorthair cats: the Mik red cell antigen. J. Vet. Int. Med. 2007;21(2):287-292. https://doi.org/10.1892/0891-6640(2007)21[287:anrbgi]2.0.co;2
- Davidow EB, Blois SL, Goy-Thollot I, et al. Association of Veterinary Hematology and Transfusion Medicine (AVHTM) Transfusion Reaction Small Animal Consensus Statement (TRACS) Part 2: Prevention and monitoring. J. Vet. Emerg. Crit. Care 2021;31(2):167-188. https://doi.org/10.1111/vec.13045
- Seth M, Jackson KV, Winzelberg S, et al. Comparison of gel column, card, and cartridge techniques for dog erythrocyte antigen 1.1 blood typing. Am. J. Vet. Res. 2012;73(2):213-219. https://doi.org/10.2460/ajvr.73.2.213
- Stiles J, Raffe MR. Hemolysis of canine fresh and stored blood associated with peristaltic pump infusion. J. Vet. Emerg. Crit. Care 1991;1(2):50-53.
- Davidow EB, Blois SL, Goy-Thollot I, et al. Association of Veterinary Hematology and Transfusion Medicine (AVHTM) Transfusion Reaction Small Animal Consensus Statement (TRACS). Part 1: Definitions and clinical signs. J. Vet. Emerg. Crit. Care 2021;31(2):141-166. https://doi.org/10.1111/vec.13044
João Araújo
DVM, BENELUX Animal Blood Bank, Braga, Portugal
Dr. Araújo received his degree from Portugal’s Universidade de Trás-os-Montes e Alto Douro (UTAD) in 2006; he spent the practical part of his final undergraduate year at the Hospital Veterinario do Porto, and he joined their Emergency and Critical Care (ECC) team after graduation, staying there until 2014. In 2016 he was elected to the Ordem dos Medicos Veterinários (OMV), the Portuguese statutory body for veterinarians, where he serves as treasurer for the Northern Council. A frequent speaker on ECC topics, he is currently Vice-President of the European Veterinary Emergency and Critical Care Society. Dr. Araújo became a founding member and CEO of the Animal Blood Bank (Benelux) in 2022, and is currently clinical director of the Hospital Veterinário do Bom Jesus in Braga, Portugal.
Maria João Dourado
DVM, Hospital Veterinário do Bom Jesus, Braga, Portugal
Maria João graduated from UTAD in 2018, having spent the practical part of her final undergraduate year at the Hospital Veterinário Central – VECC, as ECC was already one of her main areas of interest. She started her career in a small private clinic, but soon felt the need to work in emergency and critical care, and joined the emergency team at the Hospital Veterinário do Bom Jesus in mid-2018. She is a keen participant at the largest annual ECC congresses, and has completed an externship at SIAMU – VetAgro Sup in Lyon.
Other articles in this issue
Share on social media