Canine parvovirus
Written by Nicola Decaro
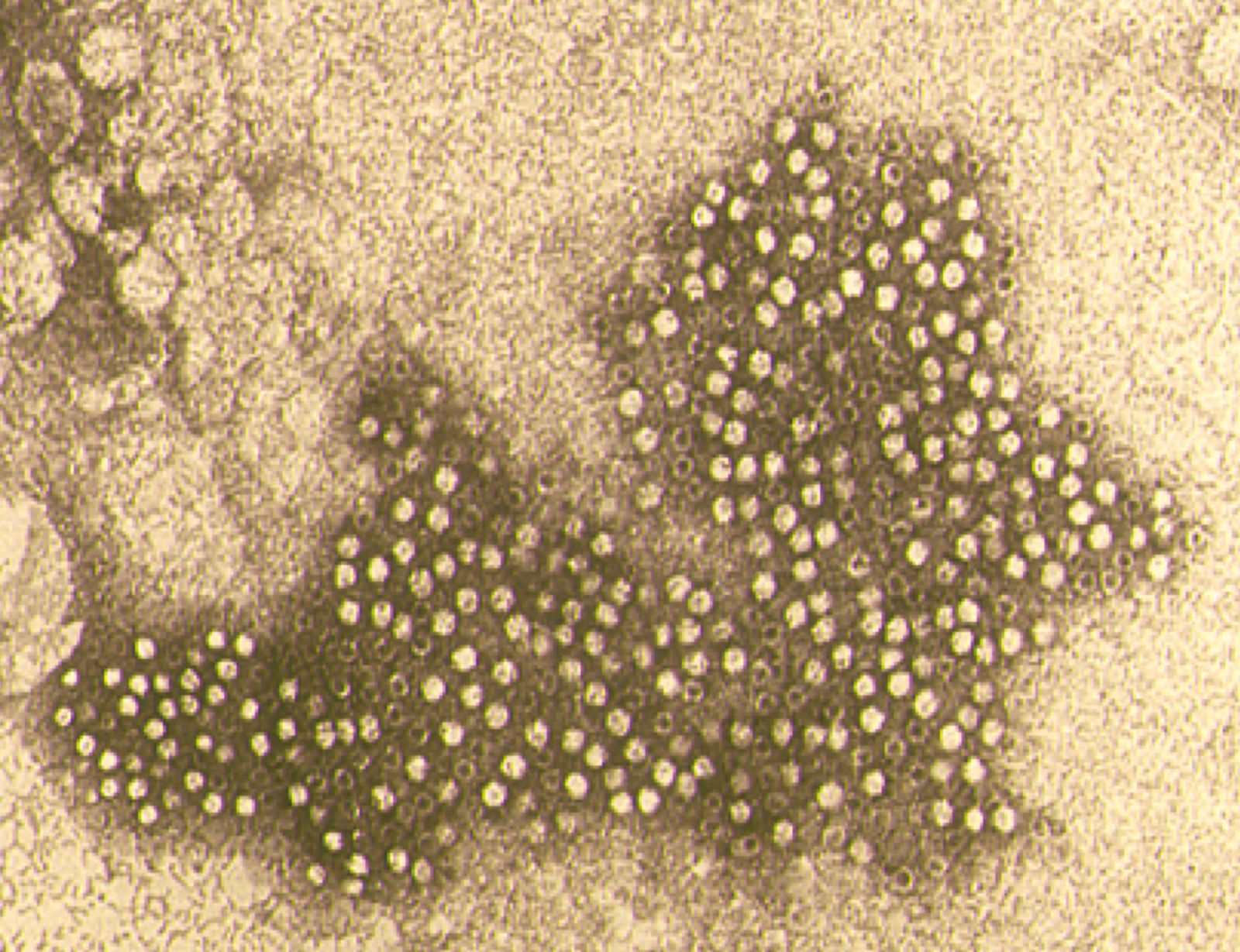
Canine parvovirus (CPV) is a small, non-enveloped virus consisting of a spherical capsid (composed of three proteins, VP1, VP2 and VP3) containing a linear, single-strand DNA molecule that encodes for two non-structural (NS1 and NS2) and two structural (VP1 and VP2) proteins.
Key points
Canine parvovirus is the main cause of acute gastroenteritis in young puppies and is found worldwide.
Three antigenic variants have completely replaced the original strain, with distribution varying according to geographic area.
In-clinic assays for diagnosis are poorly sensitive, and additional testing using PCR-based methods may be required.
Treatment is mainly supportive therapy, although several antiviral agents have been tested.
Vaccination of puppies is still the most effective strategy to control infection, despite possible interference from maternally derived antibodies and suspected mismatching between vaccine viruses and field strains.
Introduction
Canine parvovirus (CPV) is a small, non-enveloped virus (Figure 1) consisting of a spherical capsid (composed of three proteins, VP1, VP2 and VP3) containing a linear, single-strand DNA molecule that encodes for two non-structural (NS1 and NS2) and two structural (VP1 and VP2) proteins. VP2 is the major capsid protein and is responsible for virus antigenicity [1] [2]. The nomenclature of the family Parvoviridae has been recently revised, with CPV being included in the unique species Carnivore proto-parvovirus 1 along with feline panleukopenia virus (FPLV) and other related carnivore parvoviruses [3].
CPV is the main cause of acute gastroenteritis in puppies between one and six months of age. Although recognized since the late 1970s, the virus still represents a major threat to young dogs due to the severity of clinical signs and interference with active immunization by maternally derived antibodies (MDA) that can impair a vaccination program [1] [2]. Another drawback for disease control is the circulation of field variants (CPV-2a, CPV-2b, CPV-2c) that are antigenically distinct from the original CPV-2 strain, which is still contained in most commercial vaccines. There are only a few amino acid changes between CPV-2 and its antigenic variants, but it has been suggested that vaccination may offer only partial protection which may expose vaccinated dogs to infection by field strains and can sometimes lead to onset of overt disease [4] [5] [6]. The increased occurrence of the disease in adult dogs [4] [5] and the ability of the antigenic variants to infect cats, inducing clinical signs identical to feline panleukopenia [7] [8], are emerging issues that need to be confronted. This review will focus on the clinical, pathological and diagnostic aspects of CPV infection, with a brief overview of the current epidemiological situation in different countries and recommended vaccination protocols.
Epidemiology
The original CPV-2 strain emerged in the late 1970s, probably as an FPLV host variant through previous adaptation in an unknown wild carnivore species. In the early 1980s, the original virus was suddenly replaced by two antigenic variants, CPV-2a and CPV-2b, through 5 or 6 amino acid substitutions in the capsid protein VP2, and a third variant, CPV-2c, was reported in Italy in 2000 [9].
Currently the original CPV-2 strain, which is still present in most vaccine formulations, is no longer circulating in the field, whereas the three antigenic variants are variously distributed worldwide. In continental Europe the variants seem to co-circulate, with a prevalence of types 2a and 2b in Portugal, France and Belgium, types 2a and 2c in Italy, type 2a in Eastern Europe, and type 2c in the Iberian Peninsula, with the three variants equally distributed in Germany. North and South America display a high frequency of CPV-2b/2c and CPV2a/2c, respectively; in Asia and isolated islands, such as the UK, Australia and Japan, types 2a and 2b predominate [1] [2]. The few reports from Africa indicate a co-circulation of the three strains in the north of the continent and a high frequency of CPV-2a and 2b in the south [10].
CPV is able to infect domestic dogs, wolves and other wild carnivores, from which viruses intermediate between CPV-2 and CPV-2a have been frequently isolated [11]. The original CPV-2 strain could infect feline cells in vitro but not in vivo; in contrast, the new antigenic variants can infect cats, inducing a disease indistinguishable from feline panleukopenia [7] [8]. Theoretically, there is no breed susceptibility to CPV infection. Large breeds, such as German Shepherds, Labrador Retrievers, Rottweilers, Alaskan Malamutes, and Doberman Pinchers, seem to be at increased risk, but this could be due to the fact that MDA levels decline more quickly in rapidly growing large-breed puppies than with smaller-sized dogs [1] [2]. In addition, although CPV infection and disease occur mainly in puppies less than 6 months of age, severe clinical signs in adult dogs, often associated with CPV-2c infection, are being reported with increased frequency [5] [6].
The feces of infected puppies are the main source of the virus in the environment; the virus is exceptionally stable and can remain infectious for several weeks or even months. Naïve puppies are infected through the oronasal route by direct or indirect contact [1] [2].
Pathogenesis
Clinical signs and pathology
As noted above, the incubation period for the original CPV-2 strain was up to 7 days, while the new variants usually require only 3-4 days before clinical signs appear. Depending on the age and immune status of an infected dog, CPV infection can cause different clinical forms, spanning from subclinical infections to acute gastroenteritis and (very rarely) myocarditis.
Subclinical infections
Subclinical infections usually occur in puppies with intermediate levels of MDA (hemagglutination inhibition antibody titers between 1:20 and 1:80) that protect against overt disease but not against infection. Differing MDA levels among puppies of the same litter may explain why some puppies can display severe clinical forms and others show few or no signs. Adult dogs may be also infected and show no or few clinical signs, due to the higher maturity of the intestinal mucosa. Sometimes only vague signs may be noted, e.g., lethargy and loss of appetite for 2-3 days, along with transient moderate leukopenia. Subclinical infections assume particular importance in kennels and animal shelters, where the presence of healthy but infected animals may favor the spreading of the virus to other puppies [1] [2] [12].
Gastroenteric form
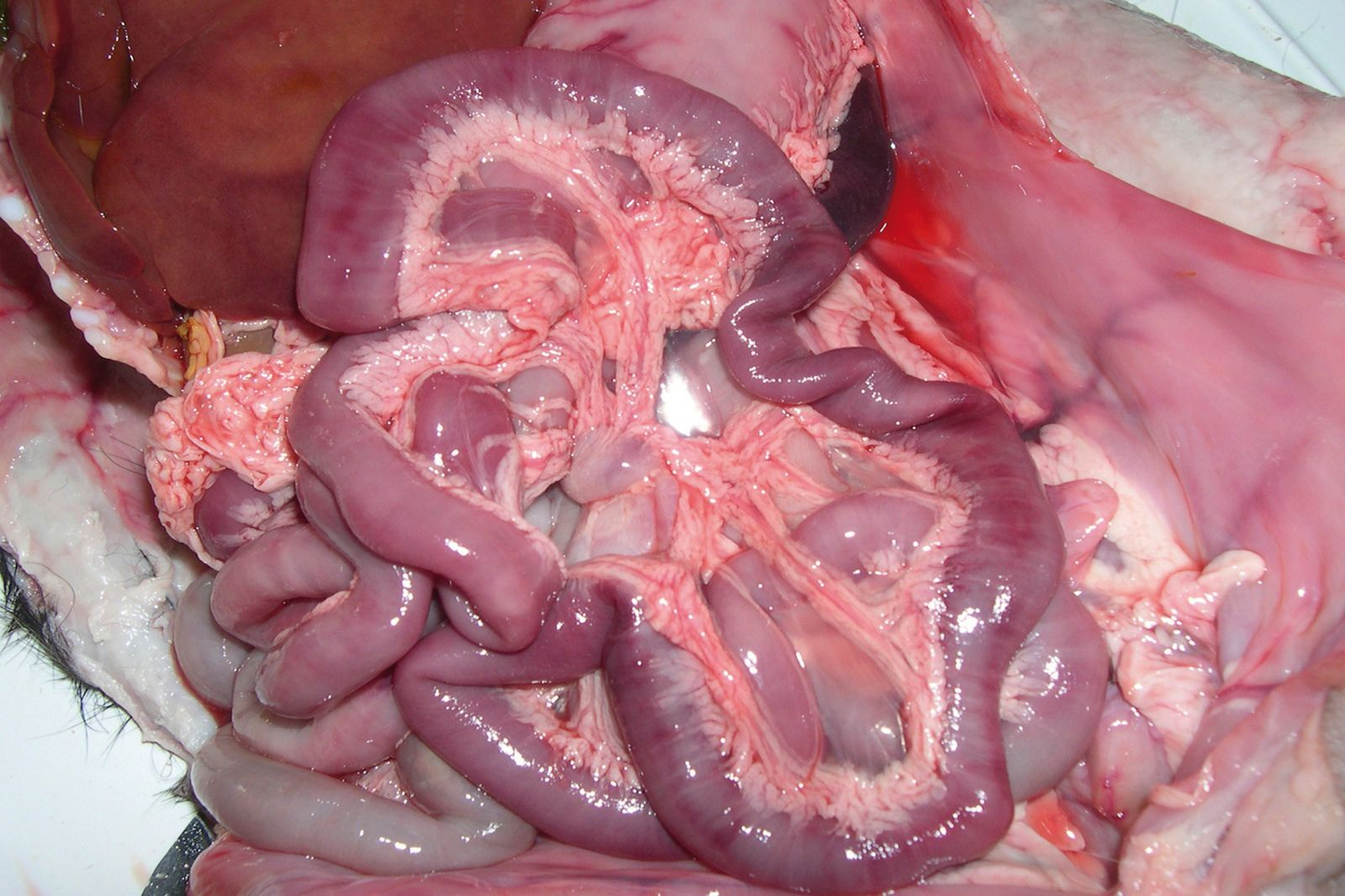
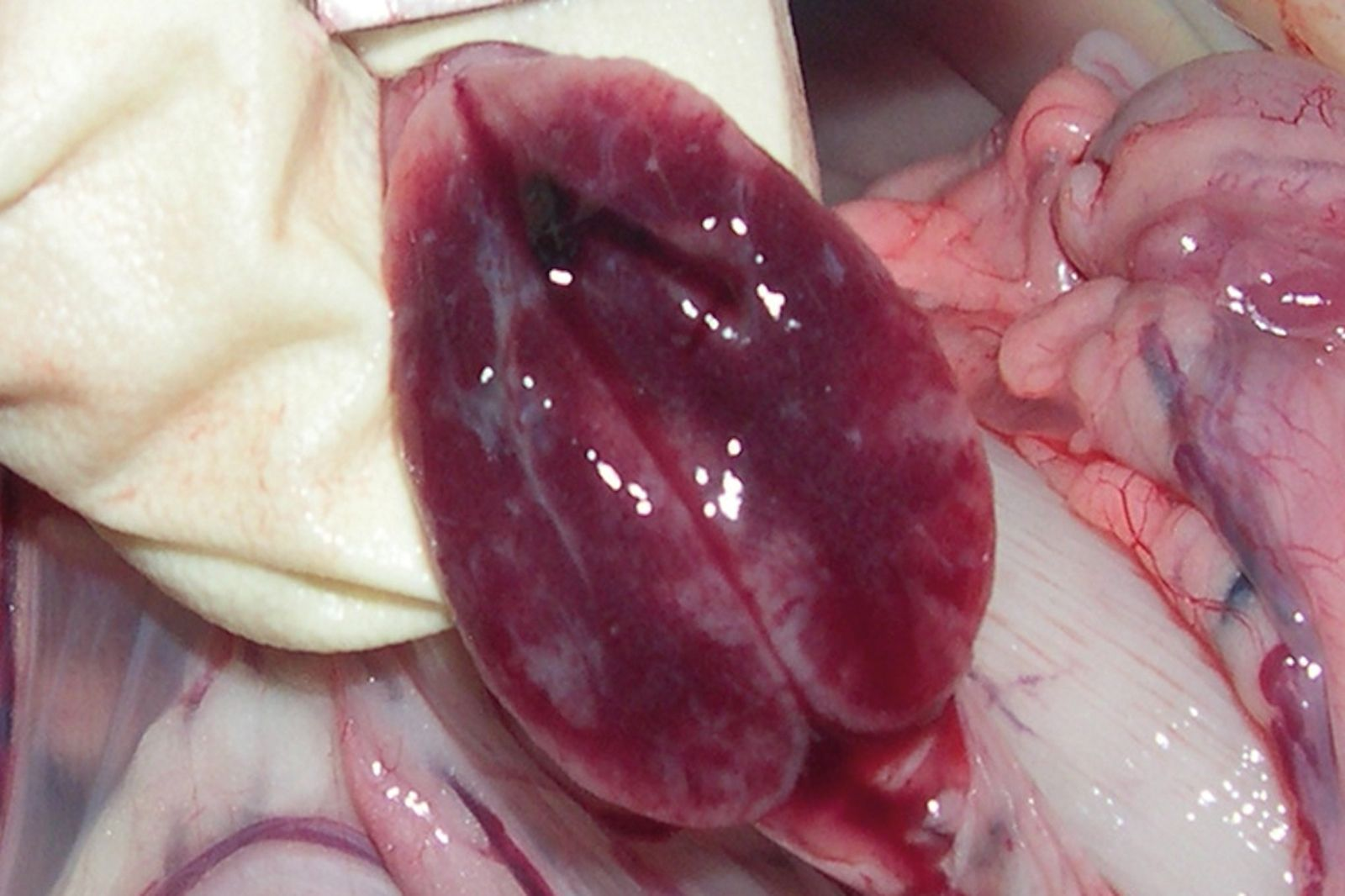
Puppies dying from CPV enteritis are extremely dehydrated. At post-mortem examination gross lesions are evident in the gastroenteric tract, mainly involving the duodenum and subsequently the jejunum. The most common finding is hemorrhagic gastroenteritis (Figure 2); the intestinal wall is usually thickened and segmentally discolored, and the serosal surface can be dark red or purple and may be covered with fibrin. The gut can be completely empty or may contain dark (often bloody) material or hemorrhagic fluid. Mesenteric lymph nodes and Peyer’s patches are enlarged and congested, often with hemorrhages scattered through the cortex and cut surface (Figure 3). Histopathologically, the small intestine is affected by multifocal crypt necrosis and intranuclear inclusion bodies, whereas extensive depletion of lymphocytes is seen in Peyer’s patches, lymph nodes, spleen and thymus. Pulmonary edema and alveolitis can be observed when there are bacterial complications [1] [2].
Myocardial form
Acute myocarditis was a common finding during the first worldwide CPV epizootics when infection involved a naïve dog population, but currently this form is only sporadically observed in the field. In fact, CPV-induced myocarditis can occur only in puppies less than 3-4 weeks of age, when the myocardial syncytium is actively replicating and is susceptible to virus replication. Nowadays, however, since the majority of bitches have been vaccinated (or exposed to the virus) and have developed a strong immune response, nearly all puppies receive MDA from their dams which protects them from parvovirus infection during the first weeks of life.
CPV myocarditis is characterized by the sudden death of infected puppies; in some circumstances, death is preceded by gastroenteric signs and a short episode of dyspnea, crying and retching. Some animals may be clinically healthy, and cardiac pathology is evident only on electrocardiography; in this situation the virus predisposes dogs to degenerative heart disease, and heart failure may develop weeks or months later. Puppies that recover from CPV myocarditis develop myocardial fibrosis. Dogs dying from the myocardial form are often in good condition and sometimes the only gross finding at post-mortem is pulmonary edema. In other cases, the affected heart exhibits flaccid walls and dilated chambers, with pale necrotic areas on the surface (Figure 4). Histopathologically, the myocardial lesions include non-suppurative myocarditis, multifocal infiltration of lymphocytes and plasma cells, and the presence of intranuclear inclusion bodies [1] [2].

Diagnostic approach
Diagnosis of CPV infection is often based simply on the presence of foul-smelling and bloody diarrhea, but it must be emphasized that other pathogens can induce similar findings and that CPV-related enteritis is frequently non-hemorrhagic. Therefore, a laboratory diagnosis is always needed to either confirm or rule out CPV infection [1] [2].
Clinical diagnosis
The presence of vomiting and hemorrhagic diarrhea, in the presence of acute leukopenia, is highly suggestive of CPV infection. However, differential diagnoses include canine distemper, infectious canine hepatitis, enteric parasitosis and other alimentary disorders. CCoV usually causes non-hemorrhagic enteritis, but under certain circumstances this pathogen can cause hemorrhagic diarrhea, and hypervirulent strains (pantropic CCoV) have been associated with systemic disease and leukopenia [13].
Virological diagnosis
Direct detection of the virus can be carried out on the feces of ill dogs or on post-mortem tissues (gut, spleen, lymph nodes). In later stages of infection, blood is the most reliable sample due to the long-term viremia. The virus has been found at high levels in all tissues, including the brain, although maximal titers are reached in lymphoid tissues [14].
There are several in-clinic commercial assays for detection of CPV in the feces. These tests detect (with equal efficacy) the three antigenic variants and even the related FPL virus. However, they are poorly sensitive, missing up to 50-60% of CPV positive samples, especially in the later stages of infection when the amount of virus shed in the feces is low and/or high CPV antibody titers in the gut lumen suppresses viable virus production [15] [16]. Hemagglutination (HA) and virus isolation tests can be performed only in specialized laboratories, and do not present significantly higher sensitivity than in-clinic testing [17]. In contrast, PCR-based methods that detect viral DNA are very sensitive and should be employed at least where there is a high suspicion of parvovirus but the puppy is negative with in-clinic testing [18]. In addition, PCR assays have been developed to discriminate between the CPV variants [19], as well as between vaccine and field viruses [20] [21] [22], which can be useful if controversies arise between dog owners, veterinarians and vaccine companies when diarrhea occurs within a few days of CPV vaccination. In fact, commercially available vaccines contain modified-live viruses that replicate in the intestinal epithelium of vaccinated dogs; the virus is shed in the feces (albeit at low titers and for a shorter time period with respect to field strains [23]) and this can lead to detection of CPV in the feces of vaccinated dogs and misdiagnosis, when clinical signs are in fact from other enteric pathogens. Moreover, PCR assays are useful to rule out the suggestion that a vaccine virus has reverted to virulence if an animal develops acute gastroenteritis shortly after vaccination.
Serological diagnosis
Despite the existence of several assays, serological testing has no diagnostic value. In fact, specific serum antibodies may be unrelated to active CPV infection if the dog has been vaccinated or had previous exposure to the virus. However, serological assays are useful to assess a dog’s immunological status with respect to CPV before and after vaccination, and by identifying the decline in MDA an assay can help calculate when a puppy can be vaccinated without interference from MDA. Serological testing is also essential to assess whether a dog has responded to vaccination or not. The most commonly used serological test is hemagglutination-inhibition (HI), which requires specialized personnel and substrates, but only virus neutralization (VN) can detect protective antibodies, and this latter test has been extensively used to evaluate the cross-neutralization between vaccine and field viruses [1] [12].
Therapeutic approach
Administration of hyperimmune plasma or purified immunoglobulins may be beneficial as a prophylactic measure for puppies in contact with infected animals, but there is no evidence for its efficacy in ill puppies. In fact, by the time clinical signs appear, the virus has colonized the target tissues and antibody levels are already high. Molecules stimulating leukocyte production, such as recombinant human or canine granulocyte colony-stimulating factor, have been anecdotally reported to shorten the duration of hospitalization and increase survival rates, but further studies are needed to confirm their efficacy. In recent years, antiviral drugs have been tested for their efficacy against CPV infection; the anti-influenza drug oseltamivir may be beneficial, but further studies are needed. Research showed that recombinant feline interferon-ω reduced clinical signs and mortality only if treatment began very early after infection [1], a circumstance not reproducible in field conditions.
Vaccination
MDA interference
The main issue for CPV vaccination is the MDA which protect puppies from infection by field strains but interfere with active immunization. MDA titers depend on the level of a dam’s serum antibodies and on the amount of colostrum ingested by the puppies. Accordingly, puppies of the same bitch can have different MDA levels, and hence be susceptible to CPV infection (and active immunization) at different ages. Vaccinating puppies with high levels of MDA (HI titers >1:20) may result in lack of seroconversion due to destruction of the vaccine virus by colostral antibodies. Since only HI titers ≥ 1:80 are considered protective against infection by field strains, there is a period – the “window of susceptibility” – usually lasting 2-3 weeks, during which puppies cannot be vaccinated but can be infected and develop disease.
To prevent interference with active immunization, vaccines should be administered to puppies only after MDA has waned [1] [2]. Different strategies have been recommended to overcome MDA interference, including high-titer vaccines and intranasal vaccination [25]. Repeated intra-nasal administrations of CPV monovalent vaccines were effective in eradicating the virus from infected kennels (personal observation).
Guidelines from the World Small Animal Veterinary Association [26] recommend that a primary CPV vaccination course should not finish until 14-16 weeks of age, to ensure protection even in puppies with long-lasting MDA; the recommended protocol involves three CPV vaccine administrations in the first year of age and a booster after one year, followed by booster vaccinations every three years [1].
CPV-2 vaccines and cross-protection with the antigenic variants
Although the window of susceptibility is the main cause for active CPV circulation among vaccinated animals, there are also concerns about the complete efficacy of type 2-based vaccines against the newer antigenic variants [4] [5] [6]. Most commercially available vaccines are prepared with the old CPV-2 strain that is no longer circulating in the field, and studies have proven the lack of full neutralization of CPV field strains by antibodies elicited against the vaccine virus. There are few licensed vaccines containing the CPV-2b variant, and it would be desirable to have formulations prepared with the new 2c variant, although the three variants are able to effectively cross-neutralize each other [4].
Nicola Decaro
DVM, PhD
Dr Decaro graduated in veterinary medicine from the University of Bari and obtained his PhD degree at Utrecht University, The Netherlands. He currently serves as Associate Professor of Infectious Diseases of Animals in the Department of Veterinary Medicine at the University of Bari. He is Associate Editor for the Journal of Virological Methods, a member of the editorial boards for several international journals, and author or co-author of many papers published in international journals. His main research interests are viral infections of carnivores and ruminants.
References
- Greene CE, Decaro, N. Canine viral enteritis. In: Greene CE, ed. Infectious Diseases of the Dog and Cat, 4th ed. St. Louis: Elsevier Saunders, 2012;67-80.
- Buonavoglia C, Martella V, Pratelli A, et al. Evidence for evolution of canine parvovirus type-2 in Italy. J Gen Virol 2001;82:1555-1560.
- Allison AB, Harbison CE, Pagan I, et al. Role of multiple hosts in the cross-species transmission and emergence of a pandemic parvovirus. J Virol 2012;86:865-872.
- Decaro N, Buonavoglia C. Parvovirosi del cane. In: Bo S., ed. Manuale di Malattie Infettive del Cane e del Gatto. Milan, Abbiategrasso 2014;38-48.
- Decaro N, Buonavoglia C. Canine coronavirus: not only an enteric pathogen. Vet Clin North Am Small Anim Pract 2011;38:799-814.
- Decaro N, Martella V, Elia G, et al. Tissue distribution of the antigenic variants of canine parvovirus type 2 in dogs. Vet Microbiol 2007;121:39-44.
- Decaro N, Desario C, Beall M.J, et al. Detection of canine parvovirus type 2c by a commercially available in-house rapid test. Vet J 2010;184:373-375.
- Decaro N, Desario C, Billi M, et al. Evaluation of an in-clinic assay for the diagnosis of canine parvovirus. Vet J 2013;98:504-507.
- Desario C, Decaro N, Campolo M, et al. Canine parvovirus infection: which diagnostic test for virus? J Virol Methods 2005;121:179-185.
- Decaro N, Elia G, Martella V, et al. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 DNA in the feces of dogs. Vet Microbiol 2005;105:19-28.
- Decaro N, Elia G, Martella V, et al. Characterisation of the canine parvovirus type 2 variants using minor groove binder probe technology. J Virol Methods 2006;133:92-99.
- Decaro N, Buonavoglia C. Canine parvovirus – A review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet Microbiol 2012;155:1-12.
- Decaro N, Elia G, Desario C, et al. A minor groove binder probe real-time PCR assay for discrimination between type 2-based vaccines and field strains of canine parvovirus. J Virol Methods 2006;136:65-70.
- Decaro N, Martella V, Elia G, et al. Diagnostic tools based on minor groove binder probe technology for rapid identification of vaccinal and field strains of canine parvovirus type 2b. J Virol Methods 2006;138:10-16.
- Decaro N, Desario C, Elia G, et al. Occurrence of severe gastroenteritis in pups after canine parvovirus vaccine administration: a clinical and laboratory diagnostic dilemma. Vaccine 2007;25:1161-1166.
- Decaro N, Crescenzo G, Desario C, et al. Long-term viremia and fecal shedding in pups after modified-live canine parvovirus vaccination. Vaccine 2014;32:3850-3853.
- Mohr AJ, Leisewitz AL, Jacobsion LS, et al. Effect of early enteral nutrition on intestinal permeability, intestinal protein loss, and outcome in dogs with severe parvoviral enteritis. J Vet Int Med 2003;17:791-798.
- Martella V, Cavalli A, Decaro N, et al. Immunogenicity of an intranasally administered modified live canine parvovirus type 2b vaccine in pups with maternally derived antibodies. Clin Diagn Lab Immunol 2005;12:1243-1245.
- Day MJ, Horzinek MC, Schultz RD. WSAVA guidelines for the vaccination of dogs and cats. J Small Anim Pract. 2010;51(6):1-32.
- ICTV Virus Taxonomy 2015. Available at: http://ictvonline.org/virusTaxonomy.asp. Accessed Sep 11, 2015.
- Cavalli A, Martella V, Desario C, et al. Evaluation of the antigenic relationships among canine parvovirus type 2 variants. Clin Vaccine Immunol 2008;15:534-539.
- Decaro N, Desario C, Elia G, et al. Evidence for immunisation failure in vaccinated adult dogs infected with canine parvovirus type 2c. New Microbiol 2008;31:125-130.
- Decaro N, Cirone F, Desario C, et al. Severe parvovirus in a 12-year-old dog that had been repeatedly vaccinated. Vet Rec 2009;164:593-595.
- Decaro N, Buonavoglia D, Desario C, et al. Characterisation of canine parvovirus strains isolated from cats with feline panleukopenia. Res Vet Sci 2010;89:275-278.
- Decaro N, Desario C, Amorisco F, et al. Canine parvovirus type 2c infection in a kitten associated with intracranial abscess and convulsions. J Feline Med Surg 2011;13:231-236.
- Dogonyaro BB, Bosman AM, Sibeko KP, et al. Genetic analysis of the VP2-encoding gene of canine parvovirus strains from Africa. Vet Microbiol 2013;165:460-465.
Other articles in this issue
Share on social media